|
IN SITU TISSUE
ENGINEERING WITH INTEGRA Embryonic
Histogenesis in Regenerative Matrices Marc E. Gottlieb, MD, FACS Revision 01-a, April
16, 2015, (original), Copyright © 2015 |
||||
|
|
|
|||
|
In Situ Tissue
Engineering with Integra Embryonic Histogenesis in Regenerative Matrices This is a presentation about tissue regeneration in biologic
matrices. It focuses on the histology
of new tissue formation in the skin and tissue regeneration product
manufactured by Integra Life Sciences ( The illustration
is a woodcut engraving published 1869 in the book “Adventures in the Apache
Country: A Tour Through |
||||
|
|
|
|||
|
This work and presentation by Marc E. Gottlieb, MD comes from
his private practice of reconstructive plastic surgery in the several
hospitals of |
||||
|
|
|
|||
|
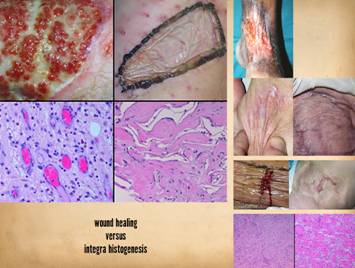
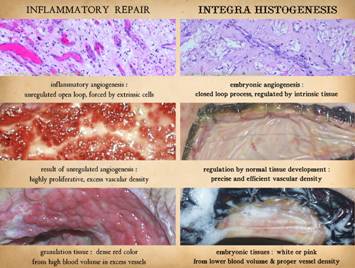
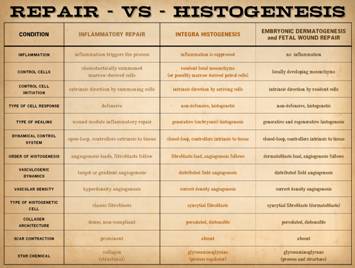
Inflammatory
Wound Healing versus Matrix Histogenesis Repair, Reconstruction, and Pathological Risks – the Inadequacies of Ordinary Surgery. Bioengineered regenerative matrices, most derived from biological
sources, appeared circa 1995. They
have since become prevalent in modern surgical practice. They allow surgeons to successfully solve
problems that otherwise, treated by conventional surgery and wound healing,
are prone to failures and complications.
This presentation does not address the general subject of biomatrices
nor their surgical techniques or spectrum of clinical indications and
results. Suffice to say that they have
significantly altered the clinical approach to many surgical problems, mainly
related to wounds and reconstruction, and they have made good results more
dependable and efficient with less risk to the patient. This presentation will instead focus on the
biological basis of their good results.
A direct comparison will be made of normal inflammatory wound healing
versus histogenesis (new tissue formation) in the matrices. The illustration
is by Pietro Berrettini (1596-1669).
Known popularly by his city of birth, Pietro da Cortona was an artist
and architect of the highest preeminence, epitomizing the High Baroque in |
||||
|
|
|
|||
|
To understand the differences between normal “post-inflammatory”
wound healing and the process that takes place within Integra and other
biomatrices, it is first necessary to review normal wound healing and how it
governs the principles of ordinary surgery.
Natural wound healing occurs by the proliferation of fibrous scar with
its capacity to contract open wound surfaces, and to “glue” together surfaces
that have been coapted. In the surgery
of repair, the methods of closing, repairing, and reconstructing
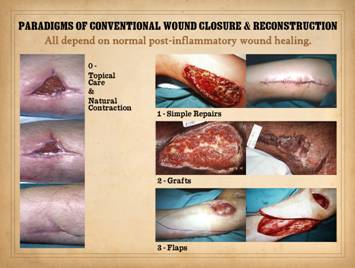
wounds and defects can all be reduced to four paradigms. The zero paradigm is to do no surgery
and instead allow the natural wound healing process to close the wound. The first operative paradigm is simple
repair by directly coapting margins of the wound. The second operative paradigm is grafts,
tissues removed from a donor area and applied to the target. They carry no blood supply of their own and
thus have stringent technicalities to keep them alive, but they are
technically simple. The third
operative paradigm is flaps, tissue transpositions that
carry their own blood supply and wound healing competence. They can be technically elaborate, but they are the most dependable option for
complex situations of exposed anatomy and impaired or pathological wounds. All four of these classic and conventional
modes of surgical wound repair depend on the normal post-inflammatory wound
healing process. In circumstances
where wound healing is impaired or dysfunctional, these surgical modes will
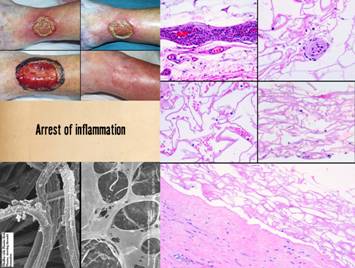
be prone to failure, complications, and persistent wounds. Left, panel of three
images showing the progressive autonomous contraction of a wound. It healed without requiring surgery. Right
top, a traumatic thigh wound for which simple coaptation of the wound
margins achieved closure. Right center, a traumatic ankle
wound. The left pane shows that the
wound has the capacity to proliferate normal wound elements and thereby is
eligible for a simple skin graft, which was applied and healed as seen in the
right pane. Right bottom, an ischial pressure ulcer which requires a flap for
closure, seen in the right pane as a large block of tissue that maintains an
attachment to the host for the sake of circulation. All four cases healed successfully due to
the health of the wound healing system in these patients. |
||||
|
|
|
|||
|
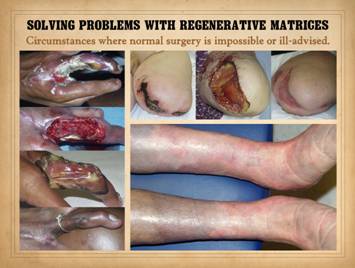
The biological and technical properties of regenerative biomatrices
allow them to succeed, to heal problem wounds and create effective
reconstructions, when normal surgical modalities are ineligible or
inadequate. Situations where
biomatrices solve complicated problems and exceed the results of conventional
surgery can be sorted into a few categories.
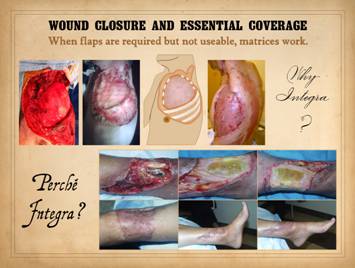
One of these is the problem of essential coverage. Essential coverage denotes circumstances of
exposed anatomy that demand coverage with living tissue, situations where
leaving the structure open would be detrimental to the safety or survival of
that structure or the patient (e.g., an exposed vital organ such as heart or
lung), or where normal wound healing cannot succeed in closing the defect
(e.g., an open joint or gliding tendon).
Flaps are the conventional solution for such situations, but when
flaps are required but not technically possible, then regenerative
biomatrices solve these problems. They
succeed in these situations because they are not alive to begin with, they
are not dependent on normal wound healing, and the mechanism of their
regeneration confers special properties and desirable attributes. Top, a series of
images of showing a chest wall reconstruction. This patient had squamous skin cancer with
neck, axillary, and chest wall invasion.
His acute presentation resulted from axillary artery rupture and
bleeding. There was no evidence of
remote metastatic disease. Resection
of this curable lesion included interscapulothoracic “forequarter” amputation
with neck dissection and chest wall resection (4 ribs). A thin alloplastic knitted mesh was used
over the chest defect to avoid possible late lung herniation, then the defect
was closed with Integra collagen-gag regenerative matrix. Its silicone outer layer is a competent
fluid and gas barrier, allowing it to keep the chest sealed without risk of
pneumothorax. As a short term skin
substitute it solved the immediate essential coverage problem with complete
safety and efficacy. For the sake of
long term stability over the defect and avoidance of a potential late
bronchocutaneous fistula, a large intercostal-epigastric flap of skin and subcutaneous
fascias was raised and delayed, the delay protected by using Integra under
the flap. At four weeks the material
was regenerated and ready for skin overgrafts, and the delay effect in the
flap was complete. At the second
surgery, the flap was lengthened and then transposed to cover the chest wall
defect and regenerated matrix, and then skin grafts were applied to the
regenerated matrix on the flap donor site, seen in the right pane a month
later. Patient has a stable healed
result and no tumor recurrence at two years of followup. Bottom,
an ankle defect following trauma, tibia fracture, orif, and hen skin necrosis
and ulceration. Several free flaps
(the conventional solution for this situation) had died, so more flaps were
ineligible. Instead, Integra
collagen-gag matrix was used to provide essential coverage over the hardware,
fracture and tendons. While serving as
an effective skin substitute in the long run, it also became the agent of
skin regeneration. It conducted new
tissue formation tangentially through the matrix, resulting in a healed wound
that required no further surgery and which preserved normal motion of ankle
and tendons. |
||||
|
|
|
|||
|
Situations where biomatrices solve complicated problems and
exceed the results of conventional surgery can be sorted into a few
categories. A second of these is the
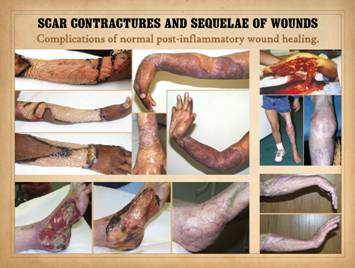
problem of scar contracture. Scars and
contractures after normal wound healing are problems of overwhelming
biological, morbid, functional, and socio-economic consequence. Scar contractures after injury and disease
can have crippling effects on the extremities and musculoskeletal structures,
deforming effects on features of vital function such as eyes and mouth, and
refractory functional effects such as stenosis of tubular organs or heart valves. Regenerative biomatrices prevent scar and
thus prevent contractures. They do
this because they suppress normal healing and induce something else, an
embryonic form of tissue generation. Early
use of these materials can preempt scar, never allowing it to occur in the
first place, and later use for reconstruction can correct scars and
contractures that have already occurred. Left upper, A young girl with severe wrist and elbow
contractures after burns. Shown is the
extremity after surgical scar excision and the placement and regeneration of
Integra collagen-gag matrix, and then the late result after skin grafting
showing normal range of motion without scar contractures. Left
lower, foot necrosis after vascular embolus and infarct and then vascular
reconstruction. Salvage of the foot
using Integra to cover bones and joints has not only healed the wound but
prevented secondary deformities of foot and ankle due to scar contractures. Right
upper, degloving injury of lower extremity, reconstructed with
Integra. Knee posture is lacking a few
degrees of full extension due to bone and joint injury, but there are no scar
or soft tissue contractures. Right lower, forearm and wrist after
clostridial myofasciitis (“gas gangrene”).
Reconstruction with Integra has prevented scars and contractures
across the wrist and has preserved full range of motion with need for
physical therapy. |
||||
|
|
|
|||
|
Situations where biomatrices solve complicated problems and
exceed the results of conventional surgery can be sorted into a few
categories. A third of these concerns
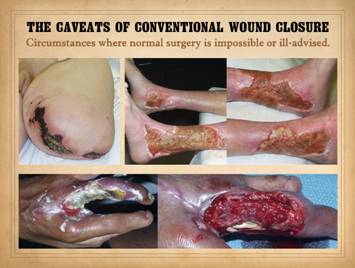
wound pathergy and the concept of a “biological superdressing”. Certain diseases put wounds and surgery at
risk of rapid infarction and ulceration via mechanisms related to vascular
disease and ischemia, coagulopathies, inflammatory states, and
autoimmunity. Pyoderma gangrenosum is
the prototype of these disorders, but any disorder resulting in wound ischemia
or inflammation has that risk.
Conventional modes of surgery that induce inflammation and depend on
normal wound healing not only incite such events but then cannot heal because
these same mechanisms disrupt normal healing.
Biomatrices are not alive when applied, so they can endure adverse conditions
that cause necrobiosis in living tissues.
They abort inflammation, so they help subside the aggravated
pathergy-prone state. The matrices
have two roles. They serve as short
term skin substitution where they not only weather the initial adverse
conditions but help subside that state.
Then, they become the agent of actual new tissue generation. They survive and heal in conditions where
conventional wound healing can never prevail. Left upper, Patient with
aorto-iliac occlusive disease and Leriche syndrome, state of the extremity
after progressive amputations beginning at the toes and progressing to thigh. Right
upper lower, a patient with Sjögren’s syndrome, legs and ankles after 40
years of chronic immunopathic ulceration and multiple failed attempts to
close with skin grafts. Lower, a patient with diabetes and
upper extremity atherosclerosis, progressive abscess, necrosis, and
incremental amputation after fingertip injury. Long finger is already missing, and ring
finger is now undergoing infarction and pending loss. Images show hand after initiating proper
wound care, and then at the time of surgery to close the wound. Results after reconstruction with Integra
are shown in next panel. |
||||
|
|
|
|||
|
The three cases shown in the last panel were all healed using
Integra collagen-gag matrix. Each of
these cases had conditions of severe arterial insufficiency and ischemia or
inflammation and autoimmunity. Each
had failed multiple prior procedures.
For the arteriopathic cases, prior surgery not only failed but it
caused more damage, avoidable amputations, and put the patients at risk of
serious systemic complications. In
their role as acute skin substitute, the regenerative matrices were not alive
when placed, so they could survive imperfect conditions while still subsiding
the inflammation. They then transitioned into their second
role as skin regenerant, thereby healing the wounds (after placement of the
final skin grafts). These patients
served as there own statistical controls, demonstrating that ordinary surgery
and wound healing failed many times, but the regenerative matrices achieved
good results on the “first try”. |
||||
|
|
|
|||
|
In Situ Tissue
Engineering with Regenerative Matrices A Paradigm of Surgical Wound Repair and
Reconstruction Independent of and Suppresses Bioengineering of new tissues has become an active subject in
the curricula and commercial activities of universities, research institutes,
and industry. Judging from research
reported, many of these activities attempt to reassemble tissue analogues by
assembly of constituent biological elements in ex vivo or in vitro bioreactors
or scaffolds. People doing such
research often tout these endeavors as a pathway to the future. That is a hopeful and meritorious abstract point
of view, but in reality, that future is already here. Regenerative biomatrices are tissue genesis
bioreactors that assemble new tissues from cellular and chemical elementals,
and they do their work in situ on the target wound. They operate independently of the post-inflammatory
wound healing process, and they also suppress inflammation and its derivative
wound healing. These are the virtues
and mechanisms of action that lead to their favorable characteristics and
clinical utility. This illustration
is also from Pietro da Cortona, Tabula XII, showing spinal nerves and some of
the peripheral and cranial nerves.
There is a certain pathos to this image, serving to remind those who
might forget that a surgeon must not do too much too soon too often, must not
operate in the face of pathergy prone conditions of ischemia and inflammation,
nor be tempted to do otherwise else his patient will pay the wages of that
indiscretion. |
||||
|
|
|
|||
|
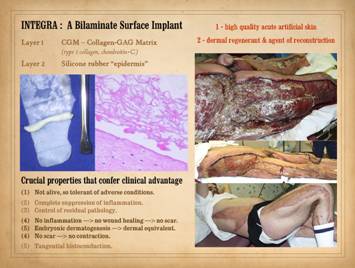
Commercially available biomatrices are mostly cadaveric dermis
from several donor species (including human).
Those products come with a connective tissue scaffold already in
place, and they have significant strength to resist sutures and biomechanical
tensile loads. Integra-CGM
(collagen-glycosaminoglycan matrix) is unique among currently available
products (2015). The working layer is
a porous spongy mashup of type 1 collagen and chondroitin-6-sulfate. It is lacking in strength and cannot be
used for structural repairs. It lacks
a pre-established connective structure, but its large “airy” pores permit the
body to readily make new structure. It
has a silicone rubber outer layer to serve as a temporary epidermis which
protects the working matrix from exposure to the ambient environment. Integra-CGM transitions seamlessly from its first to its second
role, from high quality acute artificial skin to dermal regenerant and agent
of dermal reconstruction. Alluded to
in preceding panels is that biomatrices have properties that allow them to survive
and prevail in conditions that defy normal wound healing. Those virtues can be abstracted into two
general categories, ability to arrest inflammation, and ability to suppress inflammation’s
sequel, post-inflammatory wound healing and its derivative scar. These properties are: (1) the material is not alive when placed,
so it is tolerant of adverse conditions;
(2) there is complete suppression of acute inflammation; (3) there is complete control of residual
pathology, i.e., the dysdynamical state of sustained or progressive thrombosis,
ischemia, infarction, necrosis, and inflammation that causes repetitive wound
failure; (4) no inflammation means no
normal wound healing thus no scar; (5)
the process of tissue regeneration within the matrix is nearly identical to
normal embryonic dermatogenesis, and the resulting final neodermis is
equivalent to normal dermis by many criteria, and quite unlike
post-inflammatory scar; (6) no scar
means no scar contraction; (7) unlike
skin grafts which must be in contact with and revascularized by the host for
each and every infinitesimal of its area or else die, Integra-CGM can conduct
histogenesis tangentially through the matrix, allowing it to regenerate even
when not over living material (e.g., as seen in the preceding example of
Integra over an ankle fracture and metal plate). Left, Integra with
its matrix partly rolled off of the silicone to demonstrate its bilaminar
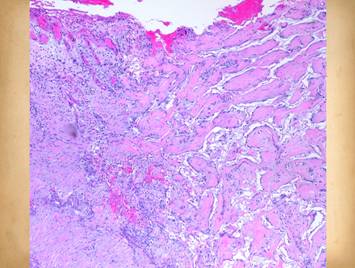
structure. The histology image shows
what the spongy matrix looks like applied to a wound, biopsy taken several
days after surgery before any cellular recognition has occurred. Although histogenetic cells have not yet
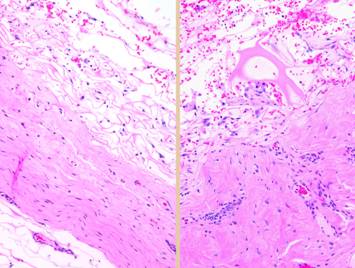
arrived, what is impressive is the lack of inflammatory cells. Right,
a patient with Group A hemolytic Streptococcus pyogenes necrotizing
fasciitis. Despite thorough
debridement and ostensible control of disease, the patient remained very
unstable in an inflammatory state. As
soon as the wounds were closed with Integra-CGM, all instabilities and signs
of acuity ceased, and the patient healed and recovered fully. The late photo shows that there are no scar
and joint contractures, and he never needed a late operation for
reconstructive purposes. |
||||
|
|
|
|||
|
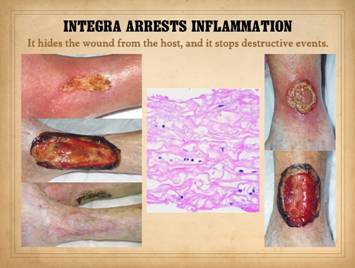
Arrest of inflammation is demonstrated here. The reasons why are explained on a later
panel. The findings seen here are
consistent from one wound or patient to the next when Integra-CGM is applied
to the wound. Very quickly, residual
erythema, edema, hyperemia, and pain subside.
It is against the “rules” of surgery to close any wound that has not
been controlled of inflammation and related adverse conditions. However, there are some wounds due to
pathological conditions where inducing complete control of inflammation is
impossible, categorical control having defied all efforts and
state-of-the-art modalities to induce control and normal wound healing. When wounds have been properly managed so
as to control inflammation to a degree seen in a normal healthy responsive
wound, then closure with Integra will resolve the remaining inflammatory
signs. Left, patient with
rheumatoid arthritis and Factor V Leiden hypercoagulable state. After all usual care for this pathological
ulcer, it remains edematous, hyperemic, inflamed, and not healing. Application of Integra has completely
subsided the inflammation. In the
bottom pane, the Integra reconstructed skin remains stable a year later. (Disease flareup resulted in a similar
situation on the posterior right ankle, seen as another piece of Integra over
the achilles). Right, patient with ulceration due to Protein S deficiency
hypercoagulability. The same pattern
is seen, complete arrest of persistent refractory inflammation once the
Integra is applied. Center, histology of Integra 10 days
after placement. The few cells seen
are “pioneer” and “transitional” cells that are the histogenetic
precursors. Never when Integra is
placed on a properly prepared wound will acute inflammatory cells
(neutrophils) appear. |
||||
|
|
|
|||
|
Control of wound healing and scar is demonstrated here. Conventional inflammation,
post-inflammatory wound healing, and scar have an intimate
interrelationship. Inflammation after
injury defends and protects the host, then cleans up debris in preparation
for healing, then triggers the healing process. The normal healing process cements the
wound together with a dense condensation of fibrous tissue, the scar. Scar is meritorious from the point of view
that it contracts and closes the wound and holds the tissues together, but
those same properties then lead to contractures. By eliminating inflammation, the secondary
process of post-inflammatory wound healing and scar is not initiated. Integra also induces tissue generation, but
in a pattern of tissue and connective deposition that is very different than
scar. The histologic architecture of the
tissue post-Integra versus post-inflammation explains the difference in wound
mechanics and clinical sequelae. Left upper, the forearm
contracture shown in a previous panel.
Integra was used to reconstruct skin after first excising the
contracted scar. Late results show no
scar, no scar hypertrophy, no contractures.
Left lower, a keloid
excised from behind the ear, skin then reconstructed preemptively with Integra
to prevent recurrent keloid. Late photo
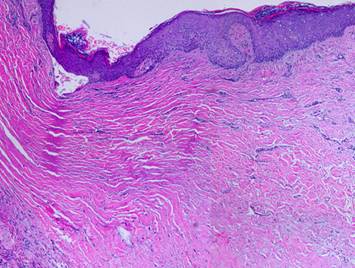
shows the area healed with no signs of scar hypertrophy. Right,
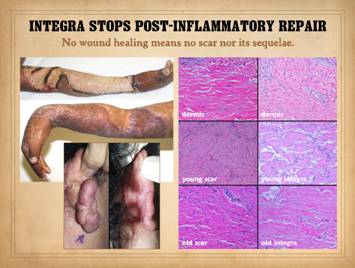
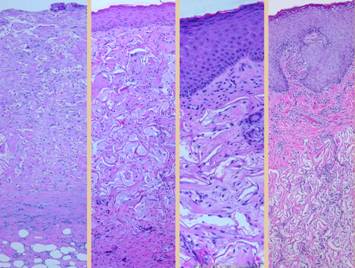
a pane of comparative histology. Top row shows normal dermis, one view
having been cut parallel to skin tension lines, the other orthogonal. Whether seen on side or on end, normal
dermis with normal elasticity and has an architecture of collagen bundles
separated or porated with interstitial spaces which give it some
deformability and pliability, typically greater in one direction than the
other. Middle row shows young scar and young Integra. The scar is dense in collagen, no spaces,
no opportunities for shifting and rolling of bundles, all oriented into
locally thick bands but without an overall uniform direction, making the scar
anisotropically stiff. In comparison,
young Integra has local fibrous foci which are separated from each other by
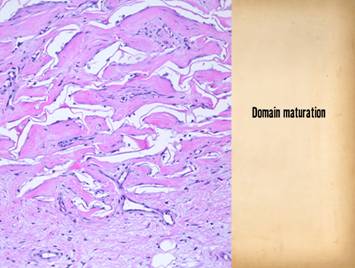
the matrix, thereby maintaining interstitial porosity and the ability of
domains to shift or distend relative to each other, a configuration and
mechanics much more like normal dermis.
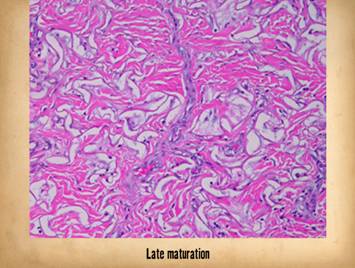
Bottom row shows scar and
Integra in phases of late maturation after many years. Both have remodeled away from their
original appearance back toward normal dermis or fascia, The difference is that young scar quickly
becomes packed with immobile excessively dense collagen, and then it takes
years to remodel back to normal stromal density, architecture, and
mechanics. Integra-CGM also takes years
to remodel back to a strictly normal appearance, but it has the fundamental
architectural and mechanical features of normal dermis right from the very
beginning. |
||||
|
|
|
|||
|
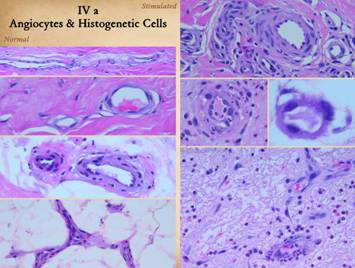
Normal post-inflammatory wound healing and its resulting scar are
very different than Integra histogenesis with its normal dermal
qualities. Despite the nominal
similarity of the two – a collection of angiocytes and fibroblasts and the
vessels and connectives they make – they are each organized in patterns and
structural mechanics that are fundamentally different from each other. These differences can be appreciated
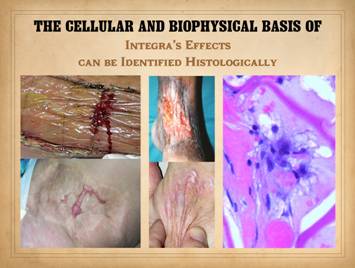
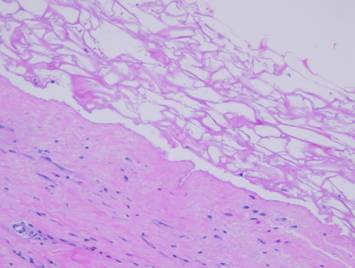
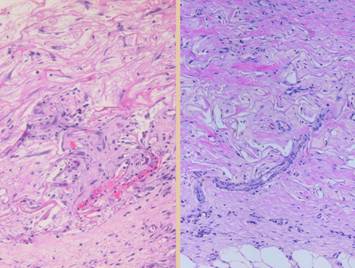
grossly and clinically and also histologically. Left upper, Integra on a
thigh. The matrix as seen through the
silicone is regenerated properly into a neodermis. In a seam between two pieces of Integra, a
small open gap has resulted in normal wound healing, recognized by the bead
of granulation tissue that has arisen.
Left lower, a similar situation
in another patient. The Integra
reconstructed skin is flat and soft and of normal color. In the center is a hypertrophic scar where
the gap between Integra edges allowed normal wound healing. Center
upper, an old trauma scar across the ankle. Scar is resisting movement and becoming
more tendinous and stiff, causing the scar to fracture and ulcerate from
normal ankle motion which in turn perpetuates the scar, inflammation, and
ulcer. Center lower, Integra reconstructed skin on the dorsum of the
hand following trauma. Just a few
weeks after skin graft placement, the neodermis is soft, compliant, and pliable
to a degree comparable to normal skin.
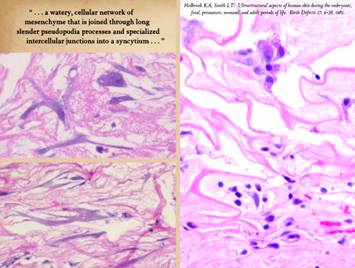
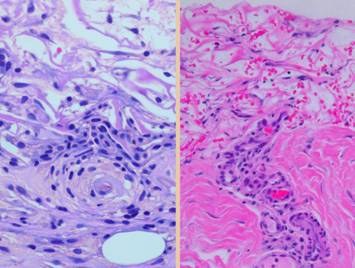
Right, a microscopic view
of regenerating Integra. The
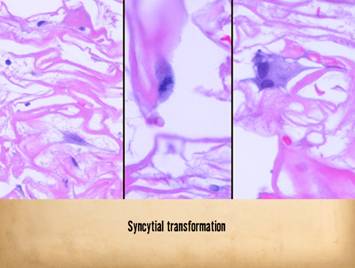
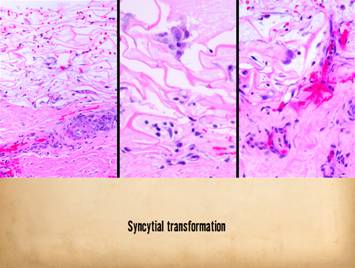
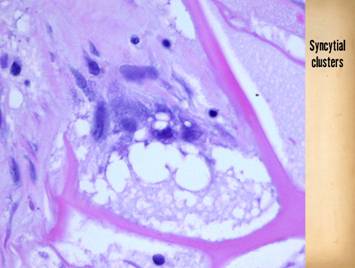
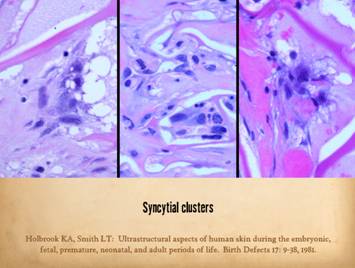
dissimilarities of scar and Integra were seen on the last panel. This view shows a syncytial cluster, a
histologic structure that is seen in embryonic dermatogenesis and in Integra
regeneration, but never in normal post-inflammatory wound healing. This structure, explained on subsequent
panels, is the basis for Integra’s biological, mechanical, and clinical properties |
||||
|
|
|
|||
|
Physiology and
Anatomy of Integra Histogenesis Comparison to Biological Properties – An Embryonic Mode of
Tissue Formation. The discussion above hints that Integra histogenesis is similar
to embryonic histogenesis, and both are quite different than inflammatory
wound healing and scar. By comparing
the microscopic appearance of these events, the basis for good results when
using regenerative matrices can be discerned. Another illustration
from Pietro da Cortona, Tabula IX, revealing the internal viscera. Like all of the Tabulæ Anatomicæ, it has a
sense of artistic pose and drama rarely matched in other anatomical studies. The art is eminently Baroque and eminently
da Cortona. It is included here to
remind that the body is highly structure.
Complex structures created during embryogenesis lead to all subsequent
functions and activities of the body.
Regenerative matrices rather than non-regenerative scar match the
embryology of normal dermis and thus match its properties. |
||||
|
|
|
|||
|
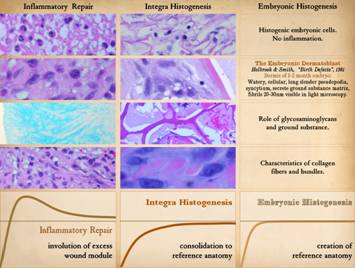
There is no better way to see the difference between normal
wound healing and Integra histogenesis than to look at them side by side, comparing
visual images of the gross and microscopic tissues that are forming. It is then easy to appreciate how different
these processes are, normal post-inflammatory wound healing and scar versus
embryonic histogenesis in a regenerative matrix (in situ bioreactor). The resulting tissues, scar versus
neodermis, are both stroma. Both have
just two cell types, angiocytes and fibroblasts. Both react to make a structural mesh of
connective proteins supplied by a network of blood vessels. Angiocytes and fibroblasts. Vessels and connectives. Despite the apparent simplicity and nominal
similarity, the two scenarios, scar versus neodermis, are profoundly
different in their histological, structural, biological, mechanical, and
functional properties. Since the
building blocks are the same, what is the difference in “programming” that
instructs these two processes to such different final structures? By examining the timewise events of normal
wound healing and matrix histogenesis, the origins of those differences can
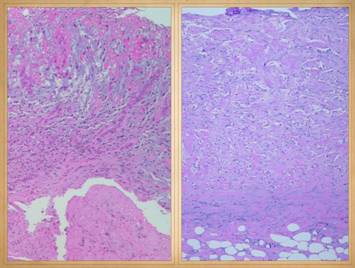
be readily observed. Left, a microscope
image of normal wound healing. The
structure shown here is the prototypical wound. Details of the structure and process will
be explained in following panels. Right, the microscopic appearance of
fully regenerated Integra-CG matrix, the details likewise to be explained in
following panels. Even without
explaining or focusing on specific details, the dissimilarity of the two can
be appreciated. Angiocytes and
fibroblasts, vessels and connectives – that is all there is to these two
tissues. However, by supplying
different “rules” or “subroutines” for the interaction and assembly of these
elements, two different biomaterials emerge.
The rules or routines are based on the circumstances,
reaction-to-injury versus embryonic regeneration. The results have very different physical properties
and implications for daily life, functional adaptations, and potential need for
ongoing medical care. |
||||
|
|
|
|||
|
Wound healing and matrix histogenesis. Both depend on the same raw elements and
cell types, yet they lead to different structures with different
characteristics and implications for
health and medical needs. Both
are important. Normal
post-inflammatory wound healing might have its deficiencies and limitations,
but it has its own strengths and virtues, and the same is true for the
matrices. Normal wound healing
provides strong, robust, rapid restoration of body integrity after injury
according to nature’s own intent.
Since the matrices are only applied when selected by a surgeon, they
are not nature’s intent, but when opted and used, matrix healing provides slow
restoration of tissue in circumstances where inflammation and pathergy prevent healing or scar creates
functional problems. Matrix healing is
like embryogenesis, so in that sense it is akin to nature’s intent, except
that nature herself turns off the process of “fetal wound healing” when we
are born. Since this presentation is coming from The images
juxtapose two bronze equestrian statues.
The |
||||
|
|
|
|||
|
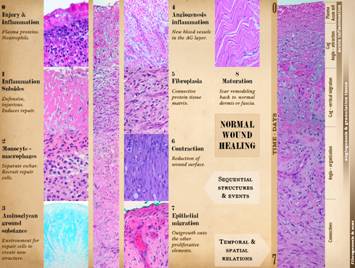
The comparison of normal wound healing and matrix histogenesis
begins with an overview of normal post-inflammatory wound healing. The anatomy of normal wound healing is
summarized in the concept of the “wound module”, the sequence of chemicals,
cells, and events which occur and self-organize to repair the stroma after
injury. Wound repair develops in
time. In an open wound, new
inflammation accumulates on the surface while repair events are occurring in
older strata below. The deeper down
you look from the surface, the older in time you are looking. When looking at wound histology, each
specimen shows its own history. At the
surface are events occurring now. As
you go deeper, you are seeing, in sequence, events that happened yesterday,
the day before, the day before that, and weeks before. Changes occur more slowly deeper down, with
less accumulation of depth, so if you plot depth (y) versus time (x), you get
a logarithmic type curve. The wound
you see under the microscope did not happen all at once. Right, the vertical
image shows the full depth of a wound from the inflammatory layer at the
surface to the organizing fibrous layer at the bottom. Scales are given to show the relative
position of anatomical strata (plasma & acute inflammation, gag’s &
angio-attraction, gag’s & vertical migration, angio-organization,
connectives) and of temporal events (acute inflammation, angiogenesis granulation tissue, fibrogenesis &
scar). The small panes show individual events within these zones. Injury and inflammation must be controlled
for repair to begin. After the wound
is closed, i.e. fully re-epithelialized, the nominal clinical endpoint of
complete repair, then the wound matures.
In between injury-inflammation and maturation, there are 7 notable and
clinically observable events: 1 -
inflammation subsides; 2 - macrophages
appear, separating eschar, and orchestrating local cells by cytokines; 3 - aminoglycan ground substance
appears; 4 - angiogenesis occurs,
visible as “granulation tissue”; 5 -
histioblasts appear, leading to fibroblasts, which make connective proteins
to hold the wound together; 6 -
myofibroblasts are another histioblast derivative, which serve to contract
the wound, responsible for much of the wound closure; 7 - epithelial growth continues until there
is a complete epithelial (ectodermal or entodermal) interface between the
environment and the mesenchyme. Each
of these events is looked at more closely in the next few panels. |
||||
|
|
|
|||
|
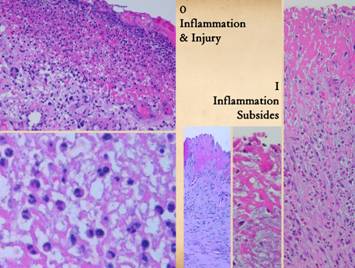
Injury, by any means, is what triggers the process of
inflammation and repair. Inflammation
is the system for recognizing and responding to an injury, the means of
defending the host, and the means of preparing for repair. It is in many ways an open loop or
auto-amplifying system, so once triggered, the response is dramatic and
intense. While meant to contain and
control threats to the host, it is inherently destructive. To the extent that inflammatory cells and
proteases contain the injury then clean up debris in preparation for repair,
the process works well. In the sick
host, with underlying disease and risk factors and limited degrees of freedom
in the wound, inflammation is the cause of pathergy, paradoxical death, and
destruction of host tissues. Histologic
features of acute inflammation include: Left, A view of the top
layer of any wound, the pink staining plasma protein and inflammatory
layer. The cells are all acute
inflammatory cells, mostly polymorphonuclear leukocytes (neutrophils) and
other leukocytes delivered from the blood.
The close up view details the inflammatory cells. Right and center, three views of wound that
have had proper care. The architecture
of the wound is the same, a plasma protein upper layer, but the cells are
gone. Inflammation has been resolved
by control of primary disease and injury, and by proper hygienic topical
care. In all of these images, the
paler or grayer staining areas below the plasma protein top layer is the
aminoglycan layer, the first stage of repair. |
||||
|
|
|
|||
|
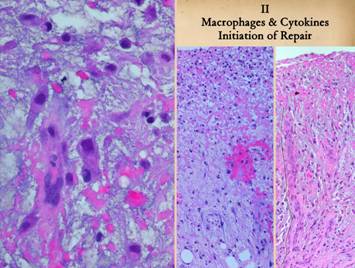
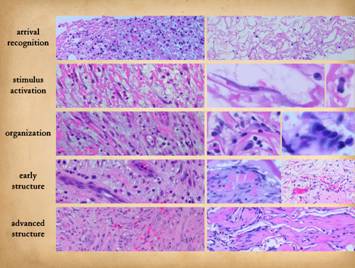
II Macrophages & Cytokines, Initiation of
Repair Inflammation brings leukocytes to the wound. They arrive in proportion to their numbers
in the blood, so neutrophils predominate, but monocytes arrive as well. Unlike neutrophils which defend then die, monocytes
have a key transitional role in the process, shifting the activity from
defense to repair. Under the influence
of platelet derived and other growth factors, monocytes transform to tissue
macrophages. They have an afferent
function as phagocytes to clean up debris from the injury and inflammation. They also have an efferent function to
initiate wound healing by release of their own cytokines and growth factors. Monocyte-macrophages are blood borne, but
the stimulated cells which then do the work of repair are local. Two cell lines must be triggered,
angiogenic cells and histioblasts. Right, close up view
near the top of a wound, at or just below the plasma protein layer. Near the top of the image are many
mononuclear cells that either appear as they did in the blood, or else are
transforming as evidenced by increasing size and cytoplasm and
nucleoplasm. In the center zone, large
mononuclear cells are mature macrophages.
In the lower zone, the organized vertical cluster of pink cells is an
angiogenic cord, a new vessel reassembling itself as angiocytes arrive. These angiocytes have migrated from vessels
below, aiming directly at the source of chemotactic stimulation, the
angiogenic cytokines made by the macrophages.
Center, a zoomed out view showing the upper
inflammatory zone, the subjacent zone of macrophage transformation, and below
that the zone of angiocyte streaming. Angiocytes
are the elongated spindle cells that are migrating from deeper layers through
the aminoglycan layer to the macrophage stimulus near the top. As they arrive, they reassemble into blood
conducting channels. In this
particular example, streaming angiocytes are abundant, but not many vessels
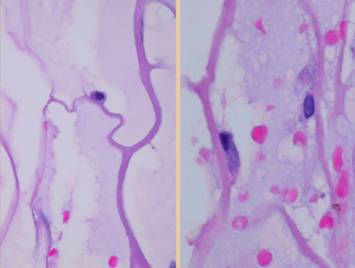
are seen yet. Right, the same view in a different wound. Angiogenesis is more mature here, with
cells mostly coalesced into new blood-conducting vessels nearly all the way
up to the inflammatory zone (one such vessel is traced with a dotted line to
demonstrate the pattern and pathway). |
||||
|
|
|
|||
|
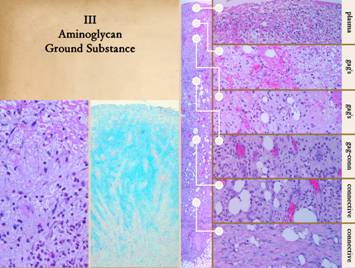
All tissues have a glycosaminoglycan (gag) ground substance, an
interstitial sol or gel that serves as the medium in which cells
“float”. These chemicals include
uronic and hyaluronic acid, chondroitin and dermatan sulfates, and
others. Mature tissues with dense
cellular parenchymas or thick fibrous stromas may have little ground
substance. Other tissues, notably
embryonic ones that have little connective protein, have a high proportion of
gag ground substance. Developing and
regenerating or healing structures need this gag environment to function and
produce a mature strong fibrous connective matrix. For the wound to heal, angiocytes will make the vascular
distribution system, and fibroblasts will make the structural framework from
connective proteins. The vessels must
arrive and assemble first in order to provide logistics and substrates for
the fibroblasts. The problem at this
point is that these regenerative cells need an environment in which to
work. At this point early in wound
healing, there is no tissue or stromal structure for them to migrate into –
that is their job, to make the new structure, to restore the stroma. Yet they also cannot migrate directly into
the plasma protein layer from whence macrophages are summoning them. Neutrophils and monocytes live in the
blood. Plasma is their home, so in a
wound they are comfortable and natural in the upper plasma layers of the
wound. In contrast, angiocytes and
fibroblasts, the cells of the fibrous stroma, do not live in nor like a
plasma environment. They need a non-plasma
pre-stromal environment conducive to histogenesis. As is the case in embryonic development,
that environment is based on aminoglycans.
Nearly all mesenchymal cells have the capacity to make ground
substance gag’s, including leukocytes and macrophages. The gag’s start to appear early in the
wound just below the plasma protein inflammatory layer. This ground substance becomes the medium,
an “ether” into which angiocytes can migrate and begin to assemble into
vessels after which the fibroblasts can start to make connective
proteins. The sub-inflammatory sub-plasma
boundary of the wound is where macrophage transformation and signaling
occur. The stratum below is the gag
layer, the zone of angio-attraction and angio-organization. Right, a vertical view
of the wound with closeup views of key features. The zones are: 1 - top layer, plasma protein,
inflammation; 2 - monocyte-macrophage
transformation and cytokine release, mainly gag’s; 3 - angiocyte streaming and loose
angiogenic organization, gag medium; 4
- organized vessels, early fibroblast proliferation, early unorganized
connective proteins filling in the gag space;
5 - histioblasts becoming young fibroblasts, fibrous stroma fills most
of the space; 6 - mature fibroblasts
with dense collagen and lamellar organization, scar. Notice the staining characteristics of
these strata. The upper plasma protein
and the lower fibrous layers stain the same because the are both composed of
proteins (different proteins, but proteins).
The aminoglycan zone in between does not pick up hematoxylin-eosin
stain very well, so it stays clear. Left, another hematoxylin-eosin view
of the upper wound layers, showing a loosely organized tissue, with cells
able to wander freely, with no fibrosis.
This is the glycosaminoglycan environment of the upper wound. H&E stain allows the location of the
aminoglycans to be inferred, but to see them directly requires alcian blue
stain. Center, alcian blue shows the tissue gag’s (it stains
carboxylated and sulfated aminoglycans such chondroitin, hyaluronan, dermatan,
but not secretory aminoglycans as found in glandular mucus; “nuclear red” counter stain shows cells). With alcian blue, the top plasma layer does
not stain, nor do the deeper connective protein layers. In between, dense blue stain is in the
sub-inflammatory macrophage layer, the streaming angiocyte layer, and the
vessel organization layer, illustrating the distribution of gag’s and ground
substance. Notice how cellular
vascular cords are clearly visualized as they rise through the wound toward
the source of stimulus. |
||||
|
|
|
|||
|
Wound healing is nothing more than the generic stroma of the
body reassembling itself after injury.
It depends on two cells, angiocytes and fibroblasts, making vessels
and connectives. The result of stromal
restoration is a foundation on which surrounding epithelium or other
parenchymal cells can grow. Vascular
and fibrous cells must come from somewhere, and that is equally true for
normal wound healing and matrix histogenesis.
Existing blood vessels in the base of the wound are the source of the
new angiocytes that will restore the stroma Stromal cells are not meant to mitose, divide, multiply in adult
life. Once an adult is fully grown,
there is no stimulus to new vessel growth since blood vessels only grow
reactively in response to tissue growth and the need for more blood supply if
tissue bulk increases. However, they
can proliferate if required or summoned to do so, such as to vascularize a
tumor or heal a wound. In their mature
adult “standby” state, angiocytes are flat or thin lamellar cells forming the
walls of blood vessels. Their flat
shape and the thickness or number of lamellae of the vessel wall are dictated
by the local biomechanics of vessel size, blood pressure, and wall shear from
flowing blood. When angiocytes have been activated, such as in a healing wound,
their origins are easily observed. The
angiocytes that migrate to a wound and establish new vessels come from
established vessels in the native tissues in the base of the wound. They are recognized because once activated
by angiogenic cytokines (such as those made by wound macrophages), vascular
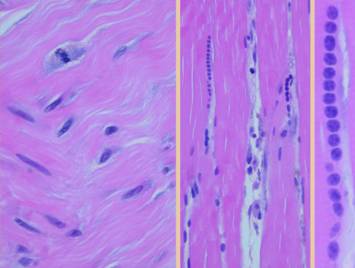
cells become large, mitotic, and migratory. Left, four images of
normal blood vessels, taken of tissues biopsied from clean healthy acute
wounds following excision of one thing or another. These views show thinner and thicker
vessels, larger and smaller, tangential, longitudinal, transverse, through
the lumen or on the surface. These
vessels are made of normal angiocytes.
Cells are flat, thin, cylinderized around the lumen. Endothelial cells are flat. Note that these are all small vessels,
capillaries and arterioles and venules.
Large vessels with a muscular media and elastic lamina are not
shown. Yet these vessels, except for
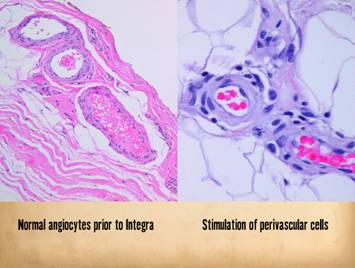
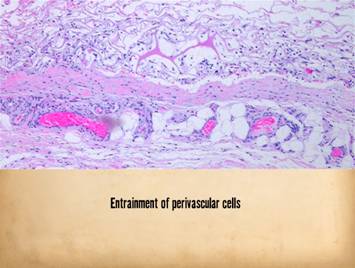
the smallest capillaries, have more than just one layer of cells. The onion-skin layers of cells around the
central endothelial layer are the vascular pericytes. These angiopericytes are the histogenesis
precursors. Under stimulation by
macrophage cytokines or other suitable stimulus, these cells will “come to
life” to heal the wound. Right, four other images taken from the actual
margins or bases of otherwise healthy wounds.
A few days after injury, vascular cells in the wound have become
hypertrophied. The angiopericytes are
thickened, with larger cell bodies and nuclei. Even the endothelial cells have become
larger and rounder and can source primitive cells. Even the smallest capillaries can respond. In the bottom image, angiocytes are seen
peeling away from the mother vessel and beginning to stream upward. As the new stroma matures and source
vessels get farther away from the leading edge of active repair, these
changes subside and the angiocytes go back to their mature structural standby
state. |
||||
|
|
|
|||
|
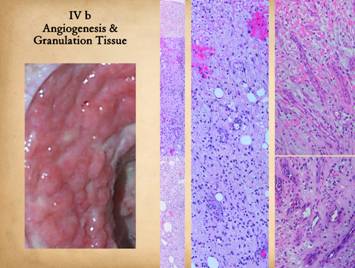
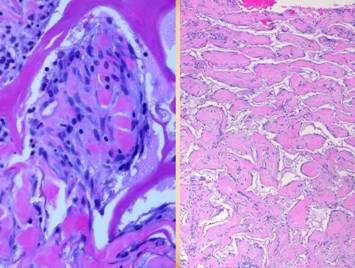
“Granulation tissue” is the one sign of a healing wound familiar
to most physicians. It is recognizable
because of its pink color and pearly texture due to excessive new blood
vessels in the aminoglycan ground substance.
The proliferation of blood vessels establishes the crucial supply
network that then permits histioblasts-fibroblasts to flourish and make
connective proteins. Angiocytes and
new vessels derive from established vessels deeper down, activated and
attracted by angiogenic cytokines n the upper strata of the wound. Left, an image of
normal healthy granulation tissue indicative of stromal proliferation and
wound healing. It has its signature
features of a pebbly red surface, dense pink color, and a “slimy” mucoid
texture. Center, this long vertical view of the wound masks the upper
plasma layer and the lower fibrous layers, highlighting the zone of
angiogenesis, the location of the distinctive features of granulation tissue. Center
right, another long vertical view.
Lumens and erythrocytes mark the location of organized new blood
vessels. Hemorrhage is present at the
junction of the gag and plasma layer.
This is where arriving angiocytes have not yet coalesced into
conducting channels, so vessels are open and inherently leaky at this level,
thus the foci of extravasated erythrocytes.
Right upper, streaming
angiocytes are highly organized, forming vessels right up to the
sub-inflammatory zone. The vessels
here all show a directional orientation, coming from old established vessels
deeper in the wound, and reaching toward the macrophages above that are
stimulating them. Right lower, organized vessels deeper in the wound. The geometry and topology of the vascular
network has become more complex here, as vessels sprout in all directions, to
accommodate the needs of fibroblastic cells which are proliferating among the
early established vessels. Note that
vessels at this level are excessive in number compared to the vascular
density of normal skin and fascias, but that vessels are otherwise mature
looking, with a single well-organized layer of cells that are no longer
enlarged or hypertrophic. |
||||
|
|
|
|||
|
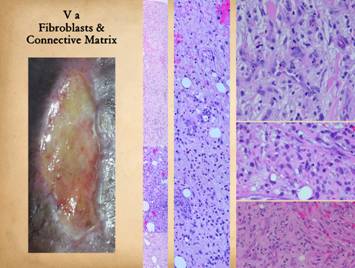
Angiocytes make vessels and establish an environment in which
later cells can proliferate. Following
that appear many new cells, coming in from strata below the new blood
vessels, which will mature into fibroblasts and myofibroblasts. As fibroblasts begin to function, they make
collagen and other connective proteins, restoring structural strength in the
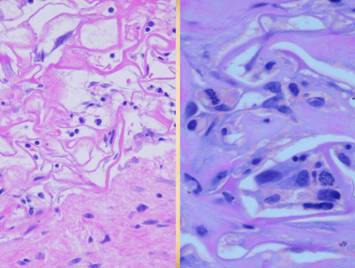
reconstituting stroma. Left, fibroplasia is
not always grossly visible in wounds or wound photos except as the final skin
scar. In this photo, angiogenic
“granulation tissue” is thin, and the deeper layer of fibrosis can be seen. Center
left, this long vertical view of the wound masks the upper layers of
plasma proteins, gag’s, and angio-organization, also the deeper layer of
maturing scar, and it highlights the zone of early fibroblasts and initial
collagen deposition. Center, another vertical view. At the top is the macrophage transformation
zone, then below is the angiocyte streaming zone. Just above middle of the picture are some
organized vessels, and between them are small cells with round nuclei. These cells become denser and more numerous
going toward the bottom. Right upper, this image is a
different wound than the adjacent vertical image, but it corresponds in depth
to the bottom of the long image. There
are organized mature vessels interspersed with the other cells. These are the histioblasts. They are starting to elongate into spindle
shapes - fibroblasts. While the matrix
is still largely aminoglycans (non-staining areas), thin strands of
eosinophilic young collagen are starting to appear. Right
middle, a little deeper, in another wound. There are vessels at bottom and upper
right, and between them histioblasts and young spindled fibroblasts are quite
dense. More of the space is occupied
by pale pink collagen. Right lower, another wound,
deeper yet. Young fibroblasts remain
dense. and the space is almost completely filled by young disorganized
collagen. As they encase themselves in
collagen, these cells become flatter and start to organize in the form of large
bundles or lamellations. |
||||
|
|
|
|||
|
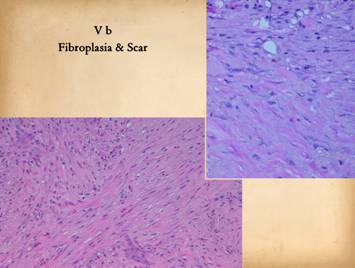
This panel is a continuation of the previous one. The previous one focused on the appearance
of histioblast-fibroblasts. This panel
focuses not on the cells but their end product, the fibrous scar. (Throughout this discussion, “collagen” is often
stated alone for convenience, but the process involves all of the connective
proteins, such as elastin and fibronectins, all of which have greater or
lesser roles in this process.) Top right, the early scar,
a level corresponding to or a little deeper than the right lower image in the
last panel. Randomly arranged young
fibroblasts are starting to become flatter and layered. They are stratified between maturing wavy
bundles of collagen. Bottom left, at yet a deeper layer,
the stratification and organization of the scar is obvious. The scar bundles are thick and dense with
collagen. Different bundles
criss-cross in different directions making the scar not only stiff, but
uniformly stiff in all directions |
||||
|
|
|
|||
|
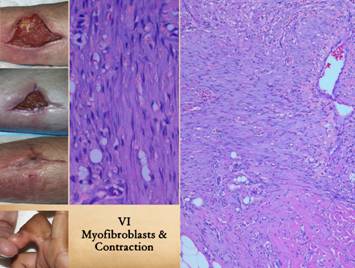
Myofibroblasts are fibroblast-looking cells which also contain
muscle proteins. Their mobility and
force generating properties allow them to pull on the wound and contract
it. They arise with the other
histio-fibroblasts. While they cannot
be discriminated with ordinary light microscopy or simple stains, their
effect is clinically very obvious. Left upper, images shown on
a previous panel demonstrating the importance of contraction for getting
wounds closed and healed. Left lower, a fibrous flexion
contracture of a finger following an old injury. This is the negative aspect of scar
contracture – physical deformities and dysfunction. In the middle image of the three images in the left upper
corner, note how the skin margins are turned inward toward the wound surface,
a common finding due to wound contraction.
Center, the microscope image
shows the wound margin subjacent to an infold of this kind. Right, a wider view of a similar
specimen. Early gag and vascular
layers are at the top (note the streaming vessels). Native fascias are below (note the adipose
cells of the hypodermis). Scar and
dense collagen are seen lower right (pink eosinophilic area). Between all of this is the darker
basophilic zone of denser, straighter, more cellular, more lamellar, more
parallel fibroblasts distinct from the other areas of fibroplasia. This is the “rubber band” that is
contracting the overlying skin and wound margins. |
||||
|
|
|
|||
|
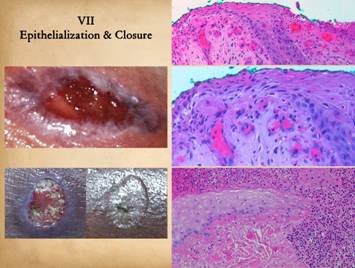
Closure of the wound means sequestration of the mesenchymal
elements underneath (the reconstituted stroma plus all native fascias) from
the ambient world without by a layer of epithelium. Epithelium cannot grow until the other
stromal elements are in place as already described, and when
epithelialization is complete, the reconstituted stroma can begin its long
process of maturation. Complete
epithelialization is the nominal endpoint of wound healing for the sake of
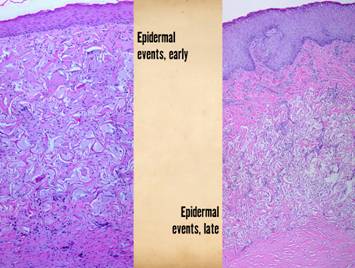
practical everyday wound management. Right upper, epidermis at
the edge of an open wound. What were
normal basal cells and acanthocytes have become primitive and migratory,
streaming outward toward a wound margin that has a suitable wound module underneath,
especially sufficient capillaries. Right middle, a close up view of the
above specimen. Migrating epithelium
bears little resemblance to its mature form, but the cells maintain contact
with each other as they spread superficially and tangentially in an elongated
flattened form. Right lower, another wound, at the edge of pressure
necrosis. The injury is two to three
weeks old. This is the edge of the
injury. Below and pink is normal
living dermis. To the right (and along
the top) is a zone of injured but living tissue, filled with acute
inflammatory cells. This area will
either heal or else separate eschar along the boundary. Above left, dark pink, is dermal necrosis,
and eschar cleavage is already occurring at the boundary. Coming in from the left is a spearhead of
migrating epidermis. It is growing
directly into the damaged interface and is responsible for eschar separation
from the margins. The cells are primitive,
but maintain a loose basal layer organization, with very thin spindle cells
at the leading edge, with rapid turnover and keratin production lifting the
eschar above. Numerous mitoses are
visible at higher powers. Left upper, epithelial outgrowth from
surrounding skin edges occurs only where granulation tissue and other wound
module elements have established a suitable foundation for epithelial cell
migration. Robust active ingrowth is
evident in the middle. Left lower, a small wound that has
healed exclusively by epithelialization rather than contraction - the margins
of the ulcerated dermis are clearly seen, even after it is healed, due to
epithelial growth over the edges and down into the crater. |
||||
|
|
|
|||
|
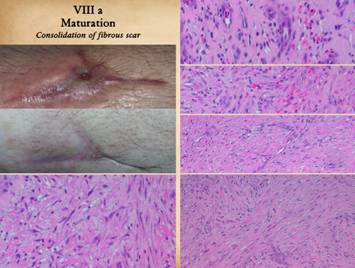
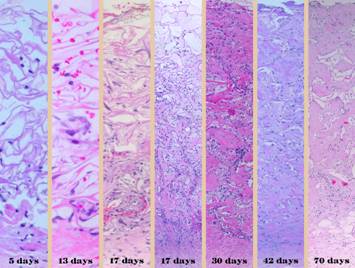
Once the wound is epithelialized and closed, there is no longer
any inflammation or wound repair stimulus, so the proliferative phase ceases. However, the strata of the wound continue
their programmed sequences until they reach a state of stability or
completion. There are three notable
events in the process of wound and scar maturation. The first is the completion of the repair
process leading to consolidation of the fibrosis. Left upper, two panes
showing a young scar at the time of complete epithelialization, and then how
it evolves into a more contracted and stronger cicatrix. Right,
the sequence of fibroplasia and its consolidation as already explained on the
preceding panels. Top, the appearance of histio-fibroblasts, with early collagen
deposition. Second, an increase in cell and collagen density, with early
lamellation and orientation of the cells and scar bundles. Third,
cell and collagen packing are denser, interlaced with mature vessels. Bottom,
new scar is at its densest, made from thick, non-compliant, highly stratified
collagen-fibroblast bundles. This is
the peak of the acute scar, having been generated in a time frame of 2 to 4
weeks after initial injury. If there
is no further inflammation or other stimulus to wound module proliferation
(which will continue to make new young scar), this peak proliferative scar will
start to modify back toward something resembling normal dermis or muscular
fascias, a process that will take weeks or months to complete. Left
lower, a point of interest. In the
other images right, the view is orthogonal to the wound surface revealing a
cross section of layered scar. The
fibrocytes appear flattened and spindle shaped. However, in this view, a tangential section
parallel to the surface through the mid zone of the healing wound, it can be
seen that the fibrous cells are actually flattened and wide. They are compressed and spread by the
tensions and geometries within the developing fibrous mesh of collagen and
connective proteins. |
||||
|
|
|
|||
|
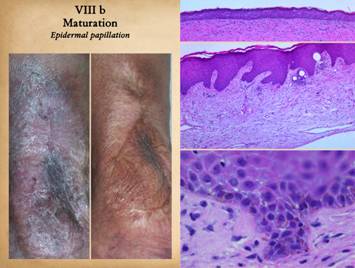
The second maturation process is the restoration of a normal
epithelium. Epithelium arrives on the
wound surface in two ways – naturally by migration from wound margins or else
by surgery (skin grafts). Either way,
the young closed wound typically has but a thin epithelium (epidermis in
these examples). After epithelial
cells arrive, they reestablish a basal stratum germinativum. As they resume their normal functions of
keratinization and epithelial cell replenishment, maturation events can be
seen. Acanthocyte proliferation
thickens the epidermis and leads to the formation of rete pegs as vascular
tufts tile the subepithelium to maintain blood supply to the thickened
lamina. The metabolically active
epidermis requires logistical support, so a lamina propria develops, the
papillary dermis. The deeper reticular
dermis is a primary structure formed embryologically or in a regenerative biomatrix. The papillary dermis is a secondary
structure, engineered by the epidermis, which does not appear until epidermis
has covered the wound. The two dermal
strata have distinctly different origins, purposes, and morphologies. Right upper, young epidermis
soon after a skin graft. The epidermis
is thin, the stratum germinativum is still immature, there is no papillation,
and no specific or differentiated histo-morphology of the subjacent scar. Right
middle, a mature regenerated epidermis.
Normal acanthosis with rete ridges and mild superficial papillomatosis
is present. Blood vessels are present
in each dermal papilla – these are the vascular tufts which supply the
epidermis. The dermal layer has two
distinct tangential zones. The upper
layer is the papillary dermis, triggered by the overlying epidermis when it
was placed on the underlying reticular layer.
The new papillary dermis is fairly normal in appearance - it may
improve further with age, but it already looks like normal native papillary dermis. The bottom reticular layer is NOT at all
like normal reticular dermis. It is
the scar from the previous open wound.
It is cellular and has lamellated collagen which is dense and
non-compliant, but with relatively thin collagen bundles compared to normal
reticular dermis - i.e. it is scar. Right lower, as epidermis matures,
other normal features appear, such as Langerhans cells and, depending on the
source of the new epithelium, melanocytes and melanin. These are all innate features of the epidermis
and epidermal-dermal interactions, and they occur independent of what had
previously happened in the mesenchymal dermis or scar or wound module
underneath. Left, two panes showing maturation of
epithelium after an ankle ulcer. Left
is a recently healed skin graft showing fragility, brittleness, accelerated
desquamation, and inconsistency of the corneum. Right is a view a year later when epidermis
has returned to normality. This
maturation corresponds to the changes seen in the histology views. |
||||
|
|
|
|||
|
The third maturation event is that which is usually meant when
talking about scar maturation - the long term involution of the scar.
The early healed wound has all of the collagen, fibroblasts, and
excessive blood vessels seen in all of the previous images. All of these elements are over abundant
compared to normal tissues. As the
healed wound ages, the excess materials are removed, and gradually the scar
takes on characteristics closer to normal skin and fascias. Left, a set of scars
from an area having had multiple operations.
Some of the scars are young, and some are old and mature. The older mature scars are pale and flat,
soft and compliant. The younger ones
are thick, stiff, and discolored from vascular plethora. Right
upper, fibroblasts, collagen, and new blood vessels at the peak of
proliferative repair with excesses of all elements. Right
middle, the “reticular layer” of skin scar after it is fully
epithelialized and the epidermis itself is healthy (same specimen as on
preceding panel). Vascular density
seems to be less, and cellularity in the collagen also seems less, compared
to their peak density in the upper image.
As the scar becomes fully matured, collagen involutes and
relaxes. Fibers and bundles become
wavy and springy, with tangential spaces or planes starting to open between the
bundles. Vessel morphology is very
mature, and the number of vessels is diminished back to a normal vascular
density, meaning that clinically the red color has faded. Fibrocyte density is much decreased. Right
lower, in the fully matured scar, herringbone patterns attest to a final
collagen configuration that is once again compliant and mobile. Vessels are sparse, and fibrocyte density
is at a minimum. While not looking precisely
like normal dermis or musculotendinous fascias, it looks very similar. |
||||
|
|
|
|||
|
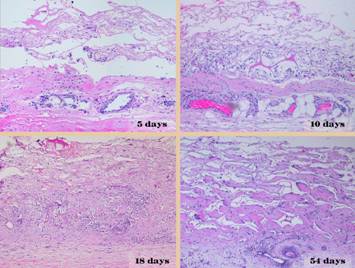
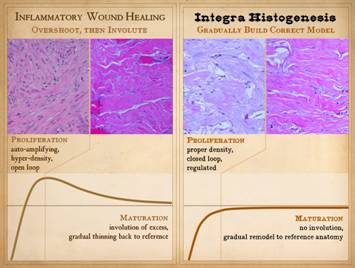
SUMMARY of Injury triggers inflammation which begets the repair
process. It is an orchestrated process
referred to as the wound module, and the significant events are: 0 - injury and inflammation trigger the process. 1 - inflammation subsides. 2 - monocytes transform to macrophages which have two jobs, the
first phagocytizing and separating eschar, the second being production of
cell stimulating cytokines to activate local histoprogenitor cells. 3 - ground substance appears so that recruited cells have an
environment in which they can function. 4 - angiogenesis begins as macrophage cytokines stimulate nearby
preexisting blood vessels. Angiocytes
stream toward the macrophages and then reorganize into blood vessels,
creating an environment in which other histioblasts can then perform their
functions. 5 - angiopericytes in old vessels also give rise to histioblasts
which come into the wound behind freshly created new vessels and then begin
to function as fibroblasts to make connective proteins which restore
mechanical stability and integrity to the wound. 6 - specialized myofibroblasts also arise, causing the wound to
contract. 7 - epithelial proliferation and migration occurs on the surface
of other established wound module elements, eventually closing the wound. 8 - once the wound is epithelialized, the wound matures, first
as the continuing consolidation of the scar and maturation of the epithelium,
followed by involution of excessive cells and proteins deposited during the
proliferative repair phase. Left, a long vertical
view of a wound, from surface and inflammatory layer to the adipose
hypodermis upon which scar is solidifying.
Right, a closer vertical
view of the proliferative sequence that establishes the scar. Center,
three views of main events in the healing of a normal wound: left
is granulation tissue from the angio-organization layer showing dense new
capillaries in non-collagenized ground substance; center,
the newly established scar of dense collagen trapping the fibroblasts that
made it; right, a scar that has completed its involution and maturation a
year after the original injury and healing. The sequential events can be observed histologically, occurring
in several distinctive zones or strata within the wound. In a normal healing wound, depth equals
history, and therefore a vertical slice of the wound represents the entire
repair process in sequence. The
recognizable strata are 1 - the top or surface layer, a coagulum of plasma proteins
populated exclusively by acute inflammatory cells. 2 - a transformation zone where monocytes are converting to
macrophages, aminoglycan ground substance replaces the plasma coagulum as the
ambient medium, and the new macrophages start to make chemotactic cytokines. 3 - a zone of streaming angioblasts, arising from subjacent
blood vessels, and migrating up through the aminoglycan ground substance
toward the source of cytokines above. 4 - a zone of angio-organization, where re-established blood
supply makes a haven for young histioblasts to proliferate and begin the
transformation to fibroblasts, where thin collagen begins to replace ground
substance. 5 - a zone of fibrous proliferation, where fibroblasts become
abundant and start to make dense connective proteins, and where wound
contraction can occur due to the effects of muscle proteinated
myofibroblasts. 6 - the fully developed scar, where fibroblasts become mature
fibrocytes, and collagen is dense and takes on a stratified architecture. 7 - epithelium grows on the surface of this wound module, from
the margins of surrounding skin, and as the epithelium closes, the wound consolidates
to mature scar and then begins the slow process of attritional maturation and
involution of excessive early elements. Inflammation and inflammatory wound repair are a coordinated
response to injury that starts with a big bang. The onset and development of inflammation
is an auto-amplifying process that dumps huge numbers of inflammatory cells
and pro-inflammatory chemicals into the wound in a very short time. The reparative process is next
characterized by rapid, highly cellular proliferation of stimulated
cells. In a healthy acute wound in an
unimpaired host, monocyte-macrophage transformation (stratum 2) is in
progress by 3-4 days after injury, angiocytes and early angiogenesis (stratum
3) can be seen grossly by 4-6 days, clinical signs of wound adhesion due to
connective proteins (stratum 4) is evident at 7-10 days, a wound able to
withstand ordinary daily mechanical loads without rupture or sutures (stratum
5) is present at 10-15 days, and a stable scar with dense collagen (stratum
6) is present in 15-20 days. Peak
consolidation of the scar is evident at 4-8 weeks, and involution and
maturational remodeling proceed from there.
Post-inflammatory wound healing is good at doing what is seen in the clinical photo. An injury occurs, the wound proliferates,
it contracts and epithelializes, and thus it is healed. There are many reasons that this can fail
resulting in chronic wounds or the need for surgery to close the wound, but
when it works properly in a healthy wound and healthy host, restoration of
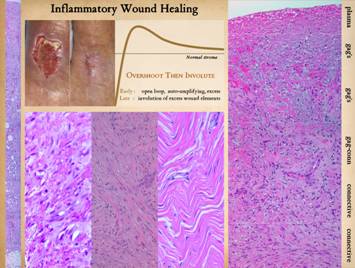
stroma and mechanical stability and a closed wound are assured. Graph: This shows the condition of the wound, some
vague nondescript measure of quality and quantity, versus time after
injury. The dotted line is a target
level representing the quality and characteristics of normal skin or stromal
tissues. The graph shows the behavior
of the repair process, beginning at the beginning with not much “stuff”. What the inflammatory wound does is to execute
its activities to excess. It deposits
large amounts of cells, vessels, and connective materials, rapidly building a
dense scar which binds the wound together, but with unfavorable
characteristics which are unlike normal skin and fascia. Only after the scar is stable and closed
does the host modify the scar, withdrawing and remodeling the excess
elements, slowly returning the scar to a state more like normal dermis and fascias. |
||||
|
|
|
|||
|
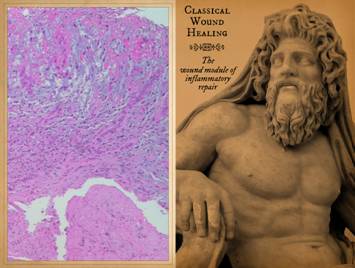
The image is a normal wound, showing its full height and
stratified architecture, from the open inflammatory layer at the top to the
fibrous scar at the base. Its
physiology, normal post-inflammatory wound healing, has now been
reviewed. It is nature’s own method, a
process that evolved early in phylogenetic history. It has been genetically conserved because
It has virtues and advantages that robustly preserve the safety, health, and
integrity of the subject. Upon injury,
this process works rapidly, generating sufficient strength to keep the wound
from rupturing from ordinary forces, on average within ten days (think about
how many days after trauma or surgery that you normally remove sutures). A healthy wound in a healthy host will heal
dependably well. Regenerative matrices
“heal” in a different way, best appreciated by direct side-by-side
comparison. The figure shown
is part of a large Roman statue from the 2nd century CE. It depicts a Roman river god. It is in the Museo Archeologico Nazionale
di Napoli (the |
||||
|
|
|
|||
|
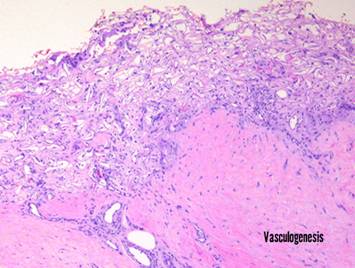
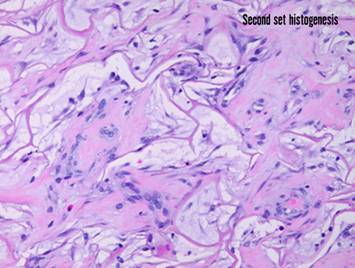
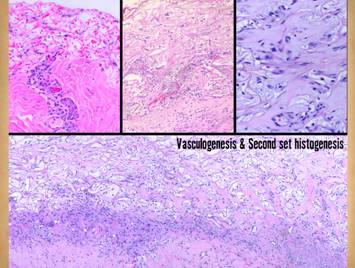
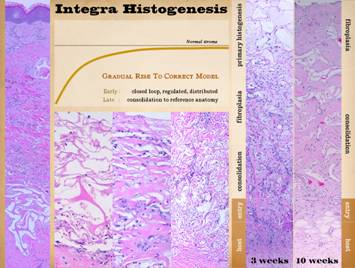
Pictured is matrix regeneration within a piece of Integra
collagen-gag matrix. It is no longer
the non-living empty matrix placed on the original wound, but a fully
restored living material. The details
of this process are now presented.
However, even without knowing the specific details, it can be
appreciated that the structure, morphology, and patterns of this regenerated
biological material are different than the microscopic structure of the
normal post-inflammatory wound. Normal
wound healing is triggered by inflammation and then evolves according to its
own “program” of how angiocytes and fibroblasts rebuild a stroma of blood
vessels and connective mesh. Integra
suppresses inflammation, and thus the normal “wound healing program” is never
turned on. Integra “heals” by a
fundamentally different mechanism analogous to embryonic tissue
generation. Its build to a state of
complete regeneration is uniform throughout the matrix, distributed rather
than stratified, and when complete, it has created a new material that has
characteristics mostly like normal dermis and quite unlike scar. The matrix coaxes the same two cells,
angiocytes and fibroblasts, to make a new tissue of blood vessels and
connective mesh in a patterned morphology that is profoundly different then
scar. The same cells, making the same
elemental components, assemble them in a completely different pattern than
wound healing and scar because the embryogenesis-and-stromal-generation
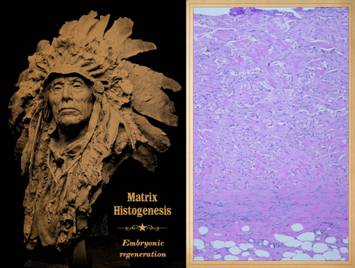
“program” is entirely different than the healing-and-scar “program”. The figure shown
is entitled “Leader of Men”, portrait of a Western tribes Indian chief. It is a bronze sculpture 2009 by western
and cowboy artist John Coleman (b. 1949) of |
||||
|
|
|
|||
|
Integra collagen-gag matrix has many favorable properties that
allow it to resolve problems not readily cured by conventional surgery that
depends on normal inflammatory wound healing.
One of its prime properties is its ability to arrest inflammation,
thereby controlling pathology and risk to the patient, but also blinding the
body to the presence of a wound. “No
wound” means no normal wound healing means no scar. How is that the collagen-gag material can
hide the wound from the host and cease inflammation? When Integra goes on a wound, normal physiological responses to
injury cease. Recognition of injury is
so severely attenuated that inflammation and its derivative events never
emerge. Integra therefore favorably
influences clinical outcomes immediately upon placement on a wound. This ability is based on several biological
properties that can be categorized as (1) its ability to immediately close a
wound, (2) to be recognized as normal tissue, (3) to suppress inflammation,
and (4) to control acute wound failure and wound pathergy. These are the properties which make Integra
dependable for critical coverage where life and limb are threatened and for
closure of pathological wounds. Left upper, chronic leg ulcer due to protein S hypercoagulable
disorder. Top left, the
ulcer as originally seen, prior to aggressive consistent topical care. Top right, after stricter
care and increased warfarin, the wound and periwound are improved, but
inflammation and active necrosis-ulceration still persist at the
margins. Bottom left,
six days after wound excision and Integra, periwound inflammation, erythema,
and edema, have completely subsided. Bottom
right, the wound is healed, a good example of a chronic refractory
ulcer due to active pathology which failed multiple prior care but healed
promptly with the collagen-gag matrix. Immediate closure of the wound and recognition as normal tissue. The composite Integra implant, matrix with
silicone, is an effective artificial skin.
The silicone pseudo-epidermis has an obvious function because it is a
thorough barrier against environmental exposure. However, it is the biocompatible spongy
matrix, looking to the body like aminoglycan ground substance, which has the
more potent beneficial effect on the wound.
When it is applied to a wound, the wound immediately stops being a
wound. It may still be an injury or
defect, but from a physiological point of view, the events which define the
usual response to injury cease. The matrix
is accepted by local cells as “self”.
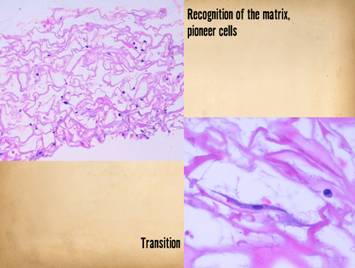
To the pioneer cells which eventually find the matrix, the material
appears to be an acellular but otherwise normal tissue. The only response triggered is a
regenerative one. This means that
inflammation and other defensive responses do not occur. Left lower, electron micrographs of matrices incubated with
platelet rich plasma. Left,
platelets adhere as expected to a collagen-cellulose matrix. Right, platelets do not
adhere to the Integra collagen-gag matrix.
Chondroitin in the matrix chemistry masks platelet binding sites on
the collagen thereby rendering the collagen invisible to platelets. If platelets do not “see” the collagen,
then they do not recognize the injury.
If they do not see the injury, then they do not turn on the
inflammatory process. No inflammation
in turn means no conventional wound healing and no scar. Inflammation and its effects are suppressed. Inflammation is the normal protective
response to injury. It also has a
central role to initiate repair which arises as injury and inflammation
subside. However, inflammation is
inherently destructive, and while it triggers wound repair, repair processes
are suppressed or damaged by persistent acute inflammation. Persistent acute inflammation is adverse to
wound healing. When inflammation
occurs reactively for identifiable reasons, e.g. an infection or a fracture
pseudarthrosis, then inflammation can be controlled by fixing the cause. When inflammation arises for erroneous reasons, e.g. Crohn’s
or rheumatoid disease, then inflammation per se must be stopped. For either scenario, until it is stopped,
physiologic wound repair will remain suppressed, and surgical wound repair is
prone to fail or even cause more damage (wound pathergy). When Integra is applied to a wound,
inflammation ceases. The gag’s in the
material hide the wound from platelets and leukocytes which aborts inflammation. They also allow the material to be
recognized as self, inviting histogenesis by pioneer stem cells which will
find the matrix. Observed
histologically, there are never inflammatory cell infiltrates in the matrix
nor even leukocyte concentration in subjacent host. Clinical signs of inflammation are
suppressed or eliminated. Pain is
often conspicuously absent after Integra, and any pre-operative periwound
erythema and edema abate rapidly (left upper images). Right, five images demonstrate absence and suppression of
inflammation, from patients having lower extremity dermatofasciectomy for
primary lymphedema (Milroy’s, praecox).
Top left, biopsy at 4 hours after fasciectomy, just
prior to placing Integra. Normal
post-traumatic thrombosis has recognized the injury, attracting
polymorphonuclear leukocytes (neutrophils) which are densely marginated in
blood vessels on the wound surface.
This is the normal response to injury, the start of inflammation. Top right, biopsy 4 hours
later after placing Integra. A blood
vessel is present at the wound surface between Integra matrix (top and left)
and normal adipose (bottom and right).
Leukocyte margination and migration are present, but not dense. Middle left, at 24 hours the
only neutrophils are a few, in proportion to the red cells that bled into the
matrix. [First three images are from
one patient, the following two are from a different patient with the same history
and surgery.] Middle right,
at 5 days, the only cells present are early histogenetic pioneer and
transitional cells. There are no
neutrophils, no lymphocytes, no plasma cells, no eosinophils, no
monocyte-macrophages. Other than some
late foreign body giant cells occurring along the silicone interface, at no
time does a defensive response ever appear in the matrix. Bottom, at 11 days, the
matrix remains mostly devoid of cells in this locale. There is still no adverse recognition or
defensive response. At least three characteristics of Integra collagen-gag matrix explain
its ability to suppress inflammation and render the wound safe from inflammation and pathergy. (1) Matrix chemistry prevents platelets
from seeing collagen, thereby preventing the thrombotic cascade to
inflammation from being triggered. (2)
The silicone artificial epidermis sequesters the wound from ambient exposure,
desiccation, bioburden, and their injurious effects. (3) The chondroitin in the matrix looks
sufficiently like normal tissue that blood borne leukocytes and lymphoid
cells that might find their way into the matrix do not recognize anything
abnormal that would trigger a defensive response, whereas histoprogenitor
cells recognize the matrix as a place to start forming new tissue. |
||||
|
|
|
|||
|
Regardless what recognizes the matrix and triggers the response,
once the response is initiated, the process is easy to track
histologically. It begins with small
round cells which pepper the matrix.
These early “pioneer cells” have characteristics that suggest they are
pluripotent stem cells, presumably derived from bone marrow. Since the Integra matrix is insoluble and
cannot issue any chemotactic signals, the discovery of the matrix by these
cells is presumably a random happenstance that occurs while they are “on
patrol” in the host tissue. Once the
matrix has been found, these cells bind to the matrix. This recognition and binding is presumably
a function of the aminoglycans in the matrix, since similar events are not
seen on pure collagen matrices. Once
bound, these cells go into “transition”.
The transitional cells start to enlarge, cytoplasm and nuclei both
getting larger in preparation for histogenic activities. Left, the matrix early after application (the time varying from
patient to patient, but approximately 5-12 days). There is no inflammatory or defensive response. The cells seen are pioneer and transitional
types. They are distributed randomly through
the matrix, including an even vertical distribution from wound surface to
silicone surface. The transitional
cells are adhering to the matrix, recognized by flattening, elongation, and early
enlargement. Right, a closeup
view of a pioneer and a transitional cell.
The pioneer cell, small and compact with minimum cytoplasm, is the origin
of the entire histogenic process within the matrix. When it recognizes the matrix and binds to
it, it has become committed to a specific cell type, equivalent to the
embryonic dermatoblast. The
transformation is recognizable as the transitional cell form, the flattened
adherent morphology that is just beginning to expand cytoplasm and
nucleoplasm in preparation for cell type specific activities. |
||||
|
|
|
|||
|
Once transformation and transition occur, the committed cell has
two functions, to replicate more cells and to start making connective protein
matrix. This is recognizable as cells
which become quite large, with large nuclei, pale reticulated cytoplasm, and
indistinct cell borders. It appears
that sister or clustered cells are coalescent, a pseudo-syncytium, thus the
title “syncytial fibroblasts”. Mitoses
are seen, and pale eosinophilic staining indicates the incipient production
of collagen. Left, a view of all of the early cell types. Small round pioneer cells are present, and
so are larger elongated transitional cells.
The largest cell in the center is a transitional cell that has matured
to its final form of the syncytial fibroblast. It is large and indistinct, with a large
nucleus and pale spongy cytoplasm. Center,
a syncytial cell with the distinctive characteristics of large size, pale
spongy cytoplasm, adherence to the matrix, and boundaries that are variably
defined. It has been caught in the act
of mitosis, seen in metaphase. Right,
a cluster of two cells which presumably began as the one in the center image,
then underwent mitosis. The concept of
a pseudo-syncytium is easily appreciated here because cell boundaries are so
indistinct as to make it seem like the two cells are fused, as would be seen
in a true syncytial cell such as a foreign body giant cell. Note that in areas adjacent to this pair,
there are pink fibers distinct from the more purplish color of the Integra
matrix. These are early collagen
fibers, indicating functional maturity of these cells. |
||||
|
|
|
|||
|
Small pioneer cells find and adhere to the matrix then transform
to a committed form recognizable as the transitional cell. They accumulate cytoplasm and nucleoplasm
then begin mitosis and proteogenesis.
These enlarged cells are equivalent to the embryonic
dermatoblast. Mitosis leads to small
clusters of cells with indistinct junctions that look under light microscopy
like a syncytium. These syncytial
fibroblasts and the clusters they spawn now begin making new tissue. The histogenic matrix at this stage has two
distinctive features. First is the
clusters themselves, undergoing mitosis and proliferation up to a certain cell
count, and second is their purpose-specific function making connective proteins, The clusters may be just two cells (even a
solitary such cell can be its own functioning cluster), or they may enlarge
up to at most 10-12 cells. The problem
is that there is not yet any vascularization into the matrix. Cells can function by diffusion of
respiratory gases and nutrients from the wound base, but without a direct
circulation, proliferation and function are diffusion limited. This limits cell count, cluster size, and
speciated functions of these cells.
Unconstrained proliferation and histogenesis will progress once
vessels arrive, but at this stage, the matrix appears to have isolated
independent clusters. Left, a broad view of
the full matrix and underlying host.
The matrix still has primitive pioneer and transitional cells, but there
are scattered enlarged cells, single, pairs, or clustered. These are the histogenic syncytial cells,
the embryonic dermatoblast. Note the
cell hypertrophy and basophilia of vessels in the subjacent host
fascias. That represents the effects
of stimulation from angiogenic cytokines, confirmation that the syncytial
clusters are feeling the stress of not having a circulation and that they are
seeking and beginning to engineer a new circulation. Center,
a closer view of the matrix and its interface with the host. In the upper area, there is a syncytial
cluster, about half a dozen cells seen.
It has not yet made substantial collagen, but this is the stage at
which early collagen becomes visible in other clusters. There are not yet any vascular cells or vessels
in the area. In the lower area at the
host-matrix interface, there is another cluster filling a pore in the
matrix. Adjacent to that, coming up
from the host are vascular cells which are in the early phase of creating a
new vessel into the matrix, quite likely stimulated by the visible cluster
given their proximity. Right, a similar view showing
enlarged syncytial cells in the matrix, including a very large 2 cell cluster
in the center. Angiohypertrophy in the
host is present, and in the right upper corner is a well formed vessel that
has infiltrated the matrix. |
||||
|
|
|
|||
|
This is a close up view of a syncytial cluster at its peak
before revascularization allows second set histogenesis (discussed on a
pending panel, “second set” is the progressive process which includes
consolidation of the matrix pores with connective proteins and evolution of
the cells to mature fibroblast morphology).
Seen in this cluster are about half a dozen syncytial cells. They are surrounded by a comparable number
of cells that have small lymphoid nuclei.
Identity of these smaller cells is uncertain, either pioneer cells not
yet transformed or else secondary or maturing fibroblast forms. However, the large cells are the focus of
this view. They have enlarged nuclei
and cytoplasm. Cytoplasm is
reticulated. The cells seem confluent
in many areas, boundaries being indistinct or intertwined. Pale pink material adjacent or between
these cells is young collagen. There
are no angioid cells, vessels, or erythrocytes. These cells are at the limit of what they
can accomplish without a direct blood supply.
Once a blood supply arrives, they will begin the second set production
of fibroblasts and connectives, and Integra pore that hey occupy, their local
domain, will begin to consolidate with collagen and a fibrous structure. |
||||
|
|
|
|||
|
Illustrated, three more views of syncytial clusters, all from
different patients, all demonstrating a consistency of the appearance and
anatomy of these cells. Left,
the same image as on the last panel.
The morphology of the cells and the cluster are highly consistent from
one subject to another. Center,
two clusters are seen. Note the big
cells, big nuclei, reticulated cytoplasm with indistinct boundaries. Two mitoses are seen (prometaphase in the
large lower cluster, metaphase in the upper group). Interspersed among the cells are areas or
streaks of young pink pale collagen. Right,
this cluster, like the others, has perhaps a half dozen syncytial cells
visible (perhaps up to a dozen when factoring in unseen sells in the three
dimensional zones above and below the image plane). Additional small cells accompany them,
presumably pioneer cells not yet transformed, or early secondary fibroblasts. (These images raise the question if the
syncytial cells, mimicking embryonic events, are recruiting additional
pioneer stem cells). The indistinct
“fuzzy” large cells seem to melt or flow into the surrounding fluids with no
hints of where the cytoplasm of one cell stops and the next starts. Large amounts of young pink collagen are
present, seen as a homogeneous non-fibrous material. This young fibrillar collagen will become
mature fibrous collagen as the “second set” of histogenesis and fibroplasia
ensues (see below). It can be seen
that although the early pioneer cells established random positions on the
Integra matrix, that their subsequent proliferation and cluster formation
must of necessity be confined to a “pore” of the Integra sponge. This establishes a domain or local architecture
to the regenerating material in which the subsequent fibrous loci that
develop around these early clusters are compact and somewhat self-contained
within each pore. To reiterate, these cells have a syncytial appearance on H&E
staining and light microscopy, but there is no insinuation here that these
are actually fused multinucleate cells.
The name “syncytial fibroblast” has been adopted to describe
morphological appearance, but functionally, these cells are the embryonic
dermatoblast. Inferences about their
unresolved microstructure are based on studies about dermal embryology and
the embryonic dermatoblast, especially the paper Holbrook
KA, Smith LT: Ultrastructural aspects
of human skin during the embryonic, fetal, premature, neonatal, and adult
periods of life. Birth Defects 17:
9-38, 1981. Investigating these
cells with electron microscopy, the paper describes “ . . . a watery,
cellular network of mesenchyme that is joined through long slender
pseudopodia processes and specialized intercellular junctions into a syncytium
. . . ”. It also clarifies that the
young collagen made by these cells is fibrillar, not fibrous. |
||||
|
|
|
|||
|
At this early stage of the histogenic process, the matrix is
populated only by scattered pioneer-transitional cells and syncytial
clusters. They are mitotically and
metabolically active, but there is a physical-logistical constraint on how
many cells can form and how much protein they can make. The constraint is that there is no vascular
supply into the young proto-tissue.
All substrate and energy supply must come by diffusion from the
underlying host. Not only is substrate
supply diffusion limited, but the cells and clusters are all competing with
each other for the same finite supply, so when the supply limit is met,
metabolic activity slows or ceases until substrate is restored. Vascularization is a space-driven event,
meaning that vessels and angiocytes do not grow autonomously or preemptively. They grow only reactively in response to a
stimulation, a “request” from cells with a need to establish a spur of the
vascular network. Angiogenic
cytokines, especially VEGF regulate this process. This is the physiology of angiogenesis and
vasculogenesis during normal embryonic growth and histogenesis. It as at this point in the Integra process
that the discongruency between metabolic needs in the proto-tissue and the
limited supply create a relative hypoxia, and each cluster responds by making
vegf. The vegf diffuses outward, and
where it impinges on nearby existing vessels, those vessels respond, cells
proliferating , mitosing, disassociating from the parent vessel, and
migrating toward the source of the stimulus.
In that regard, the origin of vascular cells and new vessels is the
same for Integra as it is for normal wound healing. Left, normal vessels in normal fascia. While any specimen could have been used for
illustration, this one is from normal native fascias at time of wound excision
and preparation just prior to Integra
placement. Like any normal vessel,
cells with thin cytoplasm and small nuclei are tightly organized into thin
tubular conduits. Right, these
vessels are in adipose fascia just below the wound-Integra interface at 10
days, when nearby syncytial clusters are putting out cytokine requests for
vascular supply. Growth factor
stimulation has caused the cells to enlarge, gaining cytoplasm and nucleoplasm. Cells are hypertrophic and hyperplastic, thicker,
rounder, and making more of themselves as seen at center lower margin where a
mitosis has been captured in anaphase.
The enlarged active cells seem to be delaminating and disaggregating
from the parent vessel. |
||||
|
|
|
|||
|
Here is a wide view of the Integra
proto-tissue and angiogenic response.
The dense fibrous stripe across center of the image is the sural
fascia of the leg upon which the Integra was placed. Below is the loose areolar fascia that
“lubricates” motion between subjacent muscles (not seen) and the sural
fascia. The host fascias are normal,
no inflammation or structural alterations, except that vessels in the loose
fascias have enlarged. Their cells
have proliferated due to angiogenic stimulation coming from syncytial cells
in the matrix. Vessels in the loose
fascia have become very hypertrophic, hyperplastic, hypercellular. Streaming of entrained cells can be seen
going from source vessels through the sural fascia into the Integra matrix. Well organized vessels are not yet seen
crossing the sural fascia nor in the Integra matrix, but erythrocytes can be
seen there, confirming blood flow. Note
how the matrix differs in its upper and lower halves. The upper half is still sparsely populated
with just the few pioneer cells and small clusters. In the lower half, where angiocytes and neogenic
vessels have entered the matrix, density has increased, less “empty” space,
more filling with tangible substance. Increased
density is partly cellular from the new angiocytes, but also because arrival
of vessels permits “second set” histogenesis wherein pores of the matrix start
to fill with collagen and a formal tissue.
Histogenesis at the base of the matrix has a patchy distribution. It is densest and best developed to right
and left where there are source vessels underneath. Toward the center where source vessels are
less, matrix lags behind. There is
still no significant physical bond between host and matrix, but it can be
seen that that is beginning along the vascular trains on the left. |
||||
|
|
|
|||
|
In this view, the process seen in the last panel has progressed. Vascular ingrowth is occurring, seen as the
dark dense cellular basophilic areas where angiocytes and vascular conduits
have crossed into the matrix. It is
around these new vessels that progressive proliferative histogenesis is
occurring. This second set
histogenesis and pore consolidation, just beginning in the last panel, has
now increased, with greater density of the matrix due to filling by cells and
pink eosinophilic collagen. Across the
lower tier of the matrix, new vessels and filling of the pores with connective
tissue is nearly complete. There is a
solid bond of matrix to host. In the
upper tier, the same process is occurring, but it is not yet as dense as in
the lower half. The matrix has now
transitioned from a multi-locus proto-tissue into a young confluent true
tissue. Angiohyperplasia and angio-migration are abundant in this view
but they are evolving. In the host
tissue and along the host-Integra interface, basophilia and hypercellularity
of vessels attest to a state of stimulated vascular proliferation. However, cell nuclei and cell structure at
these levels seem to be getting smaller, somewhat amorphous, and more
diffusely basophilic. This represents
involution of activity in these cells.
This is because source vessels in the host and at lower strata are no
longer feeling the effect of angiogenic cytokines. As the lower strata consolidate into a
proper tissue, they have the vessels they need, so they cease making
vegf. In the upper strata where this
process is still active, angiogenic cytokines are diffusing downward and
being intercepted by the first vessels they meet which are the neogenic
vessels in the lower strata that migrated and formed in the preceding few
days. Thus the first set of new
vessels in the lower strata are now the source of subsequent vessels for the
upper strata. As these latter vessels
reach into the upper strata, second set histogenesis can occur there, and
thus the histogenic process rises gradually through the matrix from bottom to
top. |
||||
|
|
|
|||
|
This is a closer look at the “second set” histogenesis, the
process in which individual domains in the proto-tissue develop into a
confluent true tissue. The “first set”
syncytial clusters attract blood vessels.
Once a vascular supply has been established at each cluster, then
progressive histogenesis can proceed, with continuing cell formation and
their metabolic activities to make connective matrix. This second phase of histogenesis is
characterized by more ordinary looking fibroblasts and the deposition of
fibrous rather than fibrillar collagen.
Between cells and connectives, pores in the Integra sponge fill with
organized, continuous, consolidated material. In the image, note that domains within the sponge are filling to
capacity with cells and collagen. The
process does not necessarily occur everywhere at once. Some domains are still empty or filled with
syncytial cells, others are becoming collagenized, and in others the process
is complete. Well formed angiogenic
cords (e.g. lower zone just to left of center) or canalized erythrocyte
conducting blood vessels (e.g. right lower corner) are present in the middle
of organizing areas. |
||||
|
|
|
|||
|
This panel reemphasizes the coordinated sequential events of
histogenesis from proto-tissue to real tissue. Early in the overall process, syncytial
clusters develop throughout the matrix, limited to a certain size by lack of
direct vascularization (“first set” histogenesis). They make angiogenic factors which trigger
the formation of new blood vessels which arise from host vessels under the
Integra matrix. Ingrowth of vessels
allows the syncytial clusters to resume the histogenic process resulting in
new cells and connectives which fill the local pores, i.e. “second set”
histogenesis. Since vascular ingrowth
can occur only at the Integra-host interface, it is only in the bottom
stratum that second set histogenesis appears initially. However, as vessels rise into the matrix,
they feel the angiogenic factors coming from higher strata, so they themselves
become the source of vessels to the upper zones. Whenever and wherever new vessels arise,
the early syncytial proto-tissue can begin the proliferation of second set
histogenesis which consolidates the matrix into a true confluent structurally
sound tissue. Top left, the Integra matrix is populated by syncytial
clusters, without yet any notable fibroplasia, density, or
consolidation. In the host tissue
below, angiohyperplasia is substantial, and a sprouting new vessel has
crossed the boundary into the Integra.
Once that blood supply has entered the matrix, second set histogenesis
can occur. Top center, in the
lower zone can be seen vessels, erythrocytes, and a general basophilia and hypercellularity
indicating the arrival of blood vessels and
the means for second set histogenesis to occur. Filling and opacification of the pores is
obvious in the zone of the revascularization.
Vascularization is also starting to reach the mid level, so connective
filling of the matrix is also evident there, though not as advanced or
consolidated as in the bottom level. The
top level of the matrix is still empty, only pioneer-transitional “first set”
cells awaiting the arrival of vessels.
Top right, a closeup view of the second set histogenic
process. Erythrocyte conducting
channels and angiogenic cords are seen.
Around them, fibroplasia is filling the pores. The consolidated new tissue is still young
and somewhat amorphous, but there is a sense of the lamination, flattening,
and layering of the fibrous cells and connective mesh that will characterize
the individual domains of the more mature tissue. Interspersed with the consolidating domains
are pores in the sponge that are empty or have syncytial cells where second
set histogenesis has not yet started. Bottom,
a broad view of this process. The
boundary layer at the host-matrix interface has diffuse basophilia indicative
of angiohyperplasia and angiocyte proliferation and migration. New vessels can be tracked into the
matrix. Second set histogenesis and
matrix filling with pink eosinophilic collagen are evident in many areas,
while many pores still remain open.
The matrix is now joined to the host. Note an important difference between this and normal wound
healing. Inflammatory wound healing occurs simultaneously across the entire
surface, one phase at a time such that event after event builds as layers
upon each other. Normal wound
architecture is thus stratified by different anatomical layers representing
different sequential events in the process.
In Integra histogenesis, there is also a sequence of events, but it
does not occur layer by layer across the entire wound surface. Instead, early pioneer cells establish
themselves evenly through the matrix in 3 dimensions, and the subsequent
histogenic processes occur individually in each local domain. Each domain goes through the same process
such that the architecture of the whole tissue is coalescence of individual
small units, granular or “cellular” (spatial segmentations, not biological
cells) rather than laminar and stratified.
The different vertical levels of the regenerating matrix must wait
their turn for vessels to rise to meet them, so the second set histogenesis
rises like a tide through the matrix, but the vertical levels are otherwise
all the same process and architecture without physical or anatomical
stratification of the final tissue.
Compare this to building a masonry wall. Normal wound healing would start by grading
the ground followed by a layer of gravel or other drainage. Then, a cement foundation would be
poured. Next, heavy stones might be
used to provide support for the massive weight of the whole wall, but at a
higher level, the construction switches to brick. Finally, at the top, some stone finials or
decorative ironwork are added to make it look good. Each level, each stratum, is laid wholly
before the next, and each stratum is laid sequentially atop the preceding
one. The construction cannot go out of
turn. A layer can be created more
efficiently by having workers in parallel each creating simultaneously a
small section of that given layer, but that efficiency cannot be applied to
the vertical dimension such that higher layers are created concurrent with
lower layers. In Integra, a
superstructure is placed first. It
would be like building a large wood or steel or concrete frame of vertical
and horizontal members that divide the vertical space into similar small
spaces (“cells”). Since the framework
can bear the weight of the wall, there does not need to be a graduated
architecture to each layer. Each cell
can be filled with the same materials and design for a thoroughly uniform
look to the finished structure. Each
cell can be filled concurrently, limited only by available logistics. Thus, while workers are crafting the “fill
in” structure in the lower stories, the upper zones might have to wait until the
electrical and plumbing utilities reach those levels so they have the
resources they need to do the same job, but once they get their supply lines,
the process is the same. If the fill
in materials or finish were done with brick or stone, then the final result
would share certain features and esthetics of the conventional masonry wall,
but it would still be quite different.
Compared to not having a framework, the initial superstructures, for
the wall and the tissue, ensure that the construction and histogenic
processes are fundamentally different and that the final structural results
are fundamentally different in appearance and mechanical properties. |
||||
|
|
|
|||
|
Once second set histogenesis is underway, the process will rise
through the matrix until it is complete in all areas. The pores in the top level will be the last
to fill and consolidate, but once they do, there is a uniform appearance to
the entire regenerated material. Left, Integra matrix that is well into the latter phases of histogenesis,
but not yet complete. At the base, the
fusion of host and Integra is complete.
The basophilia of angiohyperplasia at that layer is now involuting as
the source of neo-angiogenesis is shifting to new vessels in at mid
level. At the bottom level of the
Integra matrix are mature new vessels, and the pores of the sponge are
consolidated with pink collagen. At
mid level in the matrix, domain filling and connective production is very
active, but the pores are not as filled or consolidated as they are in the
lower zone. In the upper zone, the
pores are empty, no second set histogenesis yet. However, there are the proper number of
pioneer and transitional cells as well as well developed syncytial clusters
that are passively inhibited from making more tissue until vessels reach them. Right, the histogenic process is
complete. Basophilia and
angiohyperplasia in the host are subsided and returning to normal fascial
architecture. The matrix is uniformly
regenerated, with all domains and levels essentially the same. The lower level seems a bit more
eosinophilic, less hyaline or amorphous, more fibrous or mature than the
upper levels. This is because the
lower levels regenerated first and have had more time to evolve, although the
difference in time between upper and lower levels is only 1-2 weeks. This fully regenerated material is ready to
receive a skin graft for epithelial restoration, the final step in the
overall regenerative or tissue engineering process. |
||||
|
|
|
|||
|
As second set histogenesis progresses, collagen and connective
mesh within each pore or domain become denser and more organized. The image here is typical of this domain maturation. Cellularity seems much less prominent as
the pores have become filled with collagen and connective mesh. Small, thin, normal mature capillaries or
pre-terminal vessels are seen, roughly one vessel per domain. The collagen and connectives seem more
fibrous and lamellar than when they first started forming. The fibroblast cells seem to have become
sparser and flatter, trapped and compressed between the laminations of the
new collagen. No vestiges of
proliferative or histogenic activity remain. The neo-tissue is solidly unified with the host. All vestiges of proliferative basophilia
have retreated as cells return to normal standby or maintenance
functions. The two domains of Integra
and host fascia seem different, yet they are very similar in key
properties. The overt difference is
that the Integra sponge disperses and reorganizes the gross morphology of the
whole tissue in comparison to the non-septated appearance of the host
fascia. However, both zones have a
comparable cell density compared to collagen or volume, comparable cell sizes
and morphology, comparable vessel density, both comparable to normal
embryonically derived tissues, neither in any way comparable to the cellular
and connective excesses of post-inflammatory wound healing and scar. Capillary density in normal mammalian tissues varies with the
metabolic requirements of the tissue, but in general, the metric is that no
cell is more than about 2-10 cells widths away from a capillary (discounting neurons
and myocytes which have fractional values, i.e. multiple capillaries per
cell), average distance about 5 cell widths.
The domains seen here, each with a central vessel and surrounding
fibroblasts trapped in collagen, would seem to fit that measure. This makes this neo-tissue comparable to
normal embryonic tissues and distinct from the vascular hyperdensity of
inflammatory wound healing. As for the apparent reduction in fibroblast cellularity, an
obvious question is whether cells are diminished in number or else smaller
compared to the peak of basophilia and apparent cellularity when angiogenesis
and second set histogenesis were beginning.
The question cannot be answered with certainty by simple episodic
biopsies, but many features suggest that cell count has not diminished
through the course of this histogenesis.
Instead, the sense of diminished cellularity comes from an involution
of fibroblast size and morphology along with increasing collagen. Recall how normal blood vessels have thin
flat small cells in their ordinary unstressed state, but subjected to angiogenic
growth factors they accumulate cyto- and nucleoplasm for the sake of extraordinary
metabolic and mitotic activities. When
the stimulatory stress abates, cells recoil back to a settled mature state,
once again looking small and organized.
The same is true for the fibroblasts.
At the peak of their activity, they were big cells with big nuclei. Once the mesh is formed, their job becomes
one of maintenance rather than active creation, so cell size and plasm can
retreat. Thinner flatter cells trapped
within a bulkier matrix makes cell substance seem diminished, which it is,
but cell count remains as it was when these cells were first generated. This is how histogenesis works during
embryonic conditions: feedback or
controller regulated appearance of cells, similar cell count in any domain, 100%
stability and persistence of embryonically generated cells, absence of observed
histologic necrosis, cell ghosts, cell debris, inflammatory and reactive
cells, or any other sign of apoptosis or phagocytosis. Also, once the domains have achieved initial
filling, there is a progressive thickening of the matrix (shown on subsequent
panels) due to an increase in collagen, thus cell density per spatial volume
would seem to diminish slightly for
the same cell count. As the
matrix becomes more densely collagenized and cells flatten and mature, the
matrix takes on its “regenerated” appearance.
The morphology, size, distribution, and density of the fibroblasts at
this mature stage are comparable to normal dermis and fascias, normal
embryonic structures. Not only is the final fibrous architecture comparable to normal
embryonic tissues, but it is thoroughly unlike scar. Scar is hyperdense with cells and
connectives, a consequence of the unregulated open loop nature of inflammatory
wound healing. Long thick dense
collagen bundles within scar create stresses and resist strains in all
directions, hence their undesirable non-embryonic qualities. In the Integra domains, fibrous
architecture is different. There is
the interesting question of how the collagen types within scar versus Integra
versus normal embryonic skin and fascia might differ, a question that cannot
be answered by simple light microscopy.
However, differences in collagen typing need not be invoked to explain
most of the mechanical virtues of Integra compared to scar. The micro-architecture and anatomy explains
most of the similarities to embryonic tissues. In the regenerated Integra, each focus of
the new connective mesh seems to be locally congruent to the Integra matrix,
each domain following the contours of each pore and matrix septae. Along with the lamellations of collagen and
trapped fibroblasts, each domain takes on an onion-skin appearance of
concentric layers. In three
dimensions, these layers form a closed surface (except where each domain
intersects with adjacent pores). From
a physics point of view, these are Gaussian surfaces. Assuming a uniform elastic or contractile
modulus for the tensile properties of fibroblasts, the surface integral of
each domain should be essentially zero, no net force or contractile “flux”
across each domain. Each domain may
have internal tensions generating a pressure in the center, but between
domains and across the whole neo-tissue, net tension should be zero. The
neo-tissue cannot generate forces of contraction. Furthermore, the septations and segmentation
created by the Integra matrix divide the new tissue into isolated domains so
that there is no continuity of the fibrous tissue over long distances. Thus, even if there were directional
tensile vectors in the medium, they are distributed broadly in space rather
than along one direction, thus favoring a more compliant material even if the
collagenous material itself is intrinsically stiff. Fully regenerated, the matrix has a
distinctive appearance which is quite different than normal scar. Unlike scar with its profound propensity to contract and
distort, note how collagen and cells in the Integra neo-tissue respect the architecture
of the sponge and its septae Aside
from some vertical filling or expansion as collagen accumulation matures
(like fully inflating a balloon or tire), the morphology of the sponge
remains undisturbed, without distortion, compression, crumpling or any other
deformation that might be expected if the wound was behaving like a scar with
dense cellularity, collagenization, and contraction. Furthermore, note the space between
collagen domains and the septae of the matrix. Those spaces are fixation artifacts of
drying and preparing the slide for staining.
In real life, the connective tissue locus is in contact with the
septae. However, these images reveal
that physical adherence between new connective domains and the Integra
material are weak. This permits the
material to have internal shifts, shears, and deformations that add yet
another degree of compliance to the material that is not there, cannot be
there, in the hyperdense, hypercollagenized, non-septated, non-porated scar
material of normal wound healing. |
||||
|
|
|
|||
|
Normal embryonically derived skin and fascias, dermatocytes and
fibroblasts, are programmed to make connective tissues having a certain
morphology and architecture, textures and appearances. When fibroplasia and stromal regeneration
are stressed, perturbed, or forced in non-embryonic ways, the result is a
collagenous connective tissue with different details of organization and
structure. Scar and Integra
histogenesis are two such perturbations.
However, given enough time, the fibroblasts in these neo-tissues will
gradually remodel themselves back to the normal architecture they are
supposed to have from main sequence embryonic development. The difference is in what the remodeling
process has to start with and how far it must go to achieve relative
normality. Inflammatory wound healing
has a very non-embryonic process and chemical profile (e.g. different
collagen types than in normal tissues).
Its aggressive overshoot-then-involute dynamic makes a material that
takes a long time to remodel back to normal.
The early scar is densely cellular, excessively collagenized, and
hypervascular. Modification back to
normal means substantial gradual thinning and remodeling over a period of many
months to years. Integra histogenesis has the advantage that, going through a
more embryonic process to begin with, the initial resulting neo-tissue is
already close to normal dermis or fascia by nearly all structural,
anatomical, and mechanical properties.
However, the Integra neodermis is not categorically the same as
primary dermis. Once the neodermis has
fully formed and consolidated, typically within about 30 days of placement, it
too begins the maturation process that will gradually restore it to an
appearance more thoroughly like normal skin.
However, unlike scar, the Integra neodermis does not involute. Right from the beginning, it has normal
density of cells, connectives and vessels.
As it remodels, the collagen and connective mesh is gradually restored
to a configuration closer to normal dermis, but bulk content of cells and
connectives does not seem to change very much. In the specimen shown, 1 year after
placement, the Integra matrix itself is still present. The collagen domains are losing some of their
discrete identity, becoming more confluent.
The remodeled collagen has developed large loose wavy bundles with
more space between individual fibers, very much like what is seen in normal
dermis. However, gross clinical
characteristics of the skin at one year are not much different or more mature
than they were at 2-4 months after placement, reflecting the inherent
similarity of the material at one year to its original configuration. |
||||
|
|
|
|||
|
Integra histogenesis and normal wound healing with scar are both
mesenchymal processes. “Closure” of
the wound means restoration of epithelium over the regenerated mesodermal
elements, something that cannot occur until the mesenchymal process is
complete, thereby creating a foundation on which epidermis (or other
epithelium) can migrate and adhere.
For many natural wounds and for some small Integra reconstructions,
epidermal restoration can be done by natural epithelialization, allowing the
epithelium at the wound margins to grow spontaneously. However, many Integra reconstructions are
too large for that to be practical, so thin split thickness skin grafts are
placed at whatever point the matrix seems fully regenerated, typically at about
4 weeks. Once the graft is placed,
epithelial restoration and maturation occur the same way they would for a
skin graft placed on any wound. The
early graft must stabilize, reestablish itself as a functioning proliferative
epidermis, then mature to a final thickness and epithelial turnover rate that
is normal. Histologic observation of
the process provides further insights into Integra biology and its similarity
to embryonic tissue formation. Skin graft healing over Integra or elsewhere includes, in
sequence: Transplanted keratinocytes
reorganize into a laminated structure with a well formed basal layer (stratum
germinativum). A basement membrane
forms, created by the basal cells. The
papillary dermis, a lamina propria, forms underneath as a service layer for
circulation and other support. Papillation
occurs as the basal layer expands to an area that can source the cells needed
for a dynamic stratum corneum (the need to replace desquamated old cells),
and to maintain the geometry of adequate blood supply. These events are governed by the epidermis
itself. Left, Integra matrix that is a fully regenerated neodermis capable
of receiving and accepting an epidermal graft. The skin graft has already been placed, and
it is firmly adherent to the matrix.
The basal layer is thin and irregular, still reorganizing itself into
a correct stratum germinativum.
Acanthocytes predominate, but the stratum spinosum which they
constitute, typically the bulkiest layer of the epidermis, is still thin. Deep to the graft, at the interface with
the Integra, there are no new mesenchymal elements that were not there when
the graft was placed. For now, it is
just young epidermal graft directly on regenerated neodermis. Right, a mature Integra
reconstruction at one year. The
neodermis looks as it should at that late interval, much as it did when first
regenerated but now slowly remodeling to a form a little more like native
dermis. Above it are two strata, the
epidermis and the sub-epidermis. The
epidermis began as a skin graft. It
has now fully matured with a properly organized and regenerative basal layer,
a properly thick stratum spinosum, and a stable corneum appropriately thick
for its anatomical location on the body.
The sub-epidermal layer between epidermis and the Integra neodermis is
the lamina propria that carries vessels and lymphatics to serve the
epidermis. This layer formed under the
direction of the epidermis, and it did not need an artificial scaffold to
become this normal structure. The
lamina propria is the papillary dermis.
The Integra is the reticular dermis.
Reticular dermis and Integra are both primary or embryonic
structures. The papillary dermis
lamina propria is a secondary structure.
In normal skin, the papillary and reticular dermises are sufficiently
different to be recognizable, but sufficiently similar to seem like a single
continuous structure, but they are not. |
||||
|
|
|
|||
|
A good way to appreciate the differences between ordinary
inflammatory wound healing versus histogenesis in the collagen-gag matrix is
to look at examples of both side by side.
This panel will demonstrate angiogenesis and fibroplasia and the
significant differences between the two histogenic models. Left, an examination of vascularization and vascular density in a wound
versus Integra. Left top &
bottom, the gross and histologic appearance of inflammatory
angiogenesis. In this normal wound,
granulation tissue is proliferating.
These beads are the new vessels that have organized in the aminoglycan
angio-organization stratum of the wound.
It is a saturated red color due to high density of large diameter
vessels carrying excessive blood volume.
It contrasts sharply with the pale color of surrounding normal skin. Histology corroborates the gross appearance
of large excessive vessels. Once this
wound is closed, vascular density will return to normal over months or
years. Hypervascularity is the result
of an unregulated open loop process forced by cells (macrophages) extrinsic
to the developing tissue. Right
top and bottom, the gross and histologic appearance of Integra
histogenesis. The neodermis is fully
regenerated and ready for skin grafts.
Histology shows just a few vessels (thin cords of cells, and small
transverse rings), and vessel count and blood volume are much lower than in
granulation tissue. Color (hue and
saturation) of the new tissue appears virtually identical to the adjacent
normal skin. This is because they have
equal vascular density, both densities being just exactly what is needed to
supply the tissue. Precise and
efficient network formation results because Integra and embryonic
vasculogenesis are nearly identical dynamical processes, tightly regulated
closed loops controlled by cells (syncytial fibroblasts and embryonic
dermatoblasts) that are intrinsic to the developing tissue. Unlike what happens in scar, vascular
density in the Integra is already what it should be, and this will not change
as the tissue matures. Right upper, an examination of scar and contracture versus
neodermis. Top, scar
contracture across a joint, on the dorsal ankle after a burn. Motion of the ankle (e.g. normal walking)
puts tension on the scar, causing it to undergo tendinous metaplasia which
further decreases compliance, and causing it to fracture and ulcerate which
begets more scar. This image
illustrates the nature of chronic scar and contracture, and also the kind of
situation reconstructable with Integra collagen-gag matrix. Bottom left, dorsum of a hand
following Integra reconstruction for an electrical burn. Within just a few months of placement, the
material has the pliability of normal skin without residual stiffness or
contracture. Bottom right,
compliant regenerated Integra covering the mid back (following donation of
large flaps for radiation necrosis of the pelvis). Just a few months after the reconstruction,
while other normal scars are still young, red, and stiff, the neodermis is
very deformable, wrinkling and folding normally in response to any motion or force. This property allows joints, the face, and
other mobile parts to be reconstructed without contractures. Right center, direct
comparisons of wound healing and scar versus Integra neodermis. Side-by-side differences between them are
easily seen, both during the histogenic processes and in the final resulting
tissue. Left, Integra on
the lateral thigh following necrotizing fasciitis. It is fully regenerated and ready for skin
grafts. In the seam between two pieces,
an open area has allowed some normal wound healing to occur, resulting in
granulation tissue growing through the seam (this can be avoided by
overlapping pieces by a few millimeters).
These differing areas result in different types of final tissues. Right, healed Integra at 24
months, on the flank above the hip in a 7 year old girl. It looks mostly like normal skin. There are variances due to epidermal
pigment variegation and contour depression from absence of subcutaneous
adipose, but the quality of the skin is inherently normal, soft, pliable,
free of erythema and fibrosis. The
exception is the red scar in the center, a typical hypervascular hypertrophic
scar that developed from normal wound repair and granulation tissue in a seam
gap (like the left example). In a
child, this degree of scar hypertrophy is common and can be expected to
persist for several years. The
differences between granulation tissue and Integra histogenesis, between long
lasting hypertrophic scar and rapidly matured Integra neodermis, as shown in
these two images, recapitulates everything else that can be said about the
significant biological differences between these two processes. Right lower, a histologic comparison of fibroplasia and
connective mesh in the two processes. Left,
fibroplasia in a normal wound. This is
the zone of fibrous consolidation. Densely packed fibroblasts are making thick
cords of collagenized scar. As the
process evolves, increasing connective proteins will make the scar
progressively less compliant or distensible.
These fibrous cords are multidirectional at their inception, but
subjected to tensile loads, they will reorient themselves to resist that
load, distorting features and impeding motion. Right, Integra
collagenization. Cellularity is
low. Collagen conforms to the matrix,
forming discrete packets molded within the pores of the sponge. Spaces, interruptions, and incoherence
between collagen clusters mean that the material remains more fluid and
deformable, more like normal tissue, less like scar. |
||||
|
|
|
|||
|
In this and the next panel, the histology of normal inflammatory
wound repair is seen side-by-side against Integra histogenesis. To get this first image, a biopsy was taken
from healthy Integra at four weeks (nearly regenerated). The biopsy site was left open, thus
becoming an ordinary wound. The two
processes, wound healing and Integra histogenesis, thereafter continued side
by side, each developing according to its own set of inherent dynamics. Two weeks later, a new biopsy was taken,
centered on and at right angles to the first one. The second biopsy, seen here, captures the
interface between healthy Integra and healthy normal wound healing. The two anatomies contrast boldly. The wound healing side, left,
shows normal proper inflammatory repair, with granulation tissue, scar, and
all other strata of that process. The
Integra side, right, also looks exactly normal, properly
regenerated. Despite the obvious
visual and architectural distinctions between the two tissues, remember that
both are from the same individual host, the same cell biology, the same genome,
the same fibroblasts and angiocytes.
Yet in each situation, those cells behave according to two different “programs”. Integra’s is the embryonic histogenesis
program, something that does not happen naturally after injury and ordinary
surgery. |
||||
|
|
|
|||
|
The preceding panel showed wound and Integra during early phase
healing or histogenesis. This image
shows the two processes in late stages of remodeling and maturation. Integra was used to cover a flank defect
following tumor excision. (Renal cortex
with scarred glomeruli is in the left lower corner.) This specimen was obtained one year later
during some further surgery. On the right
is one year old Integra. Original
matrix elements are still abundant, but the Integra neodermis has matured,
now looking very much like normal reticular dermis. Epidermis from skin grafts and the induced
papillary dermis are normal. On the left
overlying the kidney is scar. This was
an area of normal wound healing just beyond the edge of the Integra. Like all normal scar, it is dense,
flattened and contracted, with thick unidirectional collagen bundles. Compare this to the more open, less dense,
more isotropic (non-directional) orientation of finer collagen in the
Integra. At one year, the scar is maturing
and involuting, and thus it is starting to look again like dermis. Given sufficient time, these two maturing
materials will look progressively the same, more like normal dermis. However, seen juxtaposed, one can
appreciate why Integra behaves so much more like normal skin right from its
original regeneration. |
||||
|
|
|
|||
|
This and the next two slides show immuno staining for growth
factors of known relevance in tissue formation, reaction to injury, and wound
healing. This panel shows the presence
and distribution of VEGF (vascular
endothelial growth factor).
VEGF has mainly angiogenic and angiotactic properties, and it is
considered the dominant angiogenic factor.
It is turned on and off by oxygen tension in the tissues. It can be made by all cells. It is how cells that are not getting
sufficient circulatory support can summon the formation and arrival of a new
capillary sprout from nearby blood vessels.
It is abundant during embryogenesis, but in adulthood, it will only appear
during circumstances that promote tissue growth, other cell proliferation, or
heightened cell metabolism. Left, a view of the
host wound just prior to placement of Integra collagen-gag matrix, thus Day 0. In the top
pane, the upper half shows the upper plasma protein layer of the wound and
the lower half shows the aminoglycan and angio-streaming zone. Because the wound had good care in
preparation for closure, there are but scant inflammatory cells in the plasma
layer, so there is little vegf, although whatever cells are there, mostly mononuclear,
are staining positive. In the gag
layer, vegf is abundant, present not only in mononuclear cells near the
boundary between the two strata, but also being made by streaming angiocytes
and young reorganizing vessels. This
is as expected. The upper gag layer
has streaming angiocytes but no formal blood supply. The migration of those cells means they are
ipso facto oxygen consuming, so they need that which is in short supply, so
they too express vegf. This
auto-amplification is one of the dynamical reasons why the angiogenesis of
inflammatory wound healing is open loop and results in excessive vascular
density. The bottom pane is a close up of a mononuclear cell, probably a
macrophage, possibly one of the arriving angiocytes, but regardless, it is
metabolically active and it is making vegf to attract a blood supply. Center,
the wound at Day 17 after Integra placement.
The top pane shows that
deeper in the native tissue, there remains some residual vegf expression of
uncertain explanation. In the zone
immediately subjacent to the Integra, vegf activity is gone. This zone now has its own stable blood
supply which can source new vessels and circulation to the matrix above. The bottom
pane is a close up of a syncytial cluster within the matrix. This is the initial dermal structure that
can reach only a limited size with only a few cells before its collective
metabolic needs outstrip locally available blood supply and oxygen
tension. It needs blood supply, and it
cannot enter its second set of proliferation and fibrogenesis until that
supply is established. These cells are
expected to be making vegf at this time, and these stains confirm that. Right,
the Integra at 30 days. At this time,
the matrix is nearly filled, but the uppermost region is still sparse of
collagen and blood supply, just entering that second set proliferation and
maturation that the lower levels started 2 weeks earlier. Thus, the upper sub-silicone zone, top pane, is expected to still show
vegf, and it does. However, the lower
part of the Integra, bottom pane,
should by now be fully regenerated and organized with mature fibroblasts and
collagen pattern supplied by mature vessels having correct density. This zone no longer as any proliferative
activity, and no longer has any mismatch between cell metabolism and
available blood supply. Vegf
production should be off by now in this zone, and the absence of stain
confirms that it is. |
||||
|
|
|
|||
|
This panel shows the presence and distribution of PDGF (platelet derived growth factor). PDGF was thus named when it was first found
in platelets, but it is found in cells throughout the body. Vegf is more of a “pure function” growth
factor, strong activity for a particular signaling function but weak for
others. In contrast, pdgf may not be
exceedingly potent, not the “prime promoter” for many proliferative functions
such as angiogenesis of fibrogenesis, but it has a broad spectrum of pro-proliferative
and tactic properties that influence embryogenesis, wound healing, and other
proliferative functions. Its presence
in platelets per se has a crucial role in the early wound to transform the
blood borne monocyte to the tissue macrophage. Left, the wound at
time of Integra placement, Day 0. The
upper pane is a wide view of the plasma and gag layers, and the lower pane
shows this area at greater magnification.
New blood vessels in the angio-streaming and angio-organization strata
of a wound are inherently leaky, which is why the wound has an obligatory
plasma protein layer. They leak
platelets as well as plasma and cells, so it would be no surprise to find
pdgf in the top layer of a wound. The
images confirm dense staining for pdgf, a validation of the known biology of
the wound. There is some seemingly
random nuclear staining for pdgf, some cells have it, some do not, but the
predominant presence of pdgf is in the top plasma layer where platelets would
ordinarily degranulate. Center, at Day 17, there is some
diffuse pale staining for pdgf, but nothing in a notable concentration or
pattern. The wider view suggests, and
the zoom view confirms that light staining is occurring in residual pores or
even in the interstitium of regenerated foci, but not in any of the
cells. New vessels being generated and
reassembled during Integra histogenesis are just loose and leaky as they are
in normal wound healing, so some interstitial pdgf staining in not surprising
in areas that are still young and actively making new tissue in a new domain
or pore. The findings suggest that
pdgf during Integra regeneration is all there passively from platelet
“spill”, but not because any of the cells are signaling with it. Right,
at Day 30, the wound has three zones as seen in the pdgf view. The original wound at the base continues to
have a background blush of stain for reason uncertain. Recall that the host wound subjacent to the
Integra goes through a process of angio-hyperplasia during stimulus from the syncytial
clusters. Once vessels arrive in the
Integra, angiogenic signaling ceases, and in turn the basal hyperplasia
ceases then and involutes, evident by increasingly amorphous basophilia and
then involution of the basophilia as the tissue returns to normal. At day 30, that recovery is still active,
so perhaps something about that host tissue and vascular recovery results in
pdgf production or release for whatever reasons. The second zone is the upper half of the
Integra which now has the nominal presence of regenerated tissue, but it is
still young tissue, still consolidating, so leaky vessels there might still
be expected. Thus some patchy pdgf
stain is still seen. The middle zone
is the lower half of the Integra, fully regenerated, but also fully
consolidated or mature, so there is no pdgf, neither by platelet spill nor by
any active local cell production. The
closeup view confirms that, except for a small amount of stain in the
uppermost still consolidating area, that pdgf is absent. |
||||
|
|
|
|||
|
This panel shows the presence and distribution of BFGF (basic fibroblast growth factor). Like all growth factors involved in
mesenchymal and stromal regulation, bfgf has a spectrum of activities, but it
is more like vegf in having high potency for a main function. It has mainly transforming effects on
fibroblast progenitors such as angiopericytes in the host vessels, and then
cytogenic and cytotactic effects on fibroblasts. Left,
at Day 0, the wound has bfgf staining where expected, a little in the plasma
protein layer, and a good amount in the gag layer, consistent with the
physiology that macrophages are the first to start signaling for cells to
heal the wound. There is also some bfgf
in the vascular cords themselves that are arising through the angio-streaming
and angio-organization strata. This
suggests that fibroblasts do not just passively arrive after the angiocytes
have made vessels because blood supply and logistics have now been
established. Instead it suggests that
the new vessels may actively be attracting or transforming fibroblasts, as if
they “know” that they are responsible for getting this next cell type “on
line”. Center, at Day 17, the partially regenerated Integra and the host
tissues underneath are replete with bfgf which is present diffusely and
without apparent patterning or focal production. The bottom close up view shows that
staining is within the newly forming collagen and fibrous mesh, not just in
cells. Right, at Day 30, the intense and diffuse bfgf staining has
largely disappeared from matrix and host.
Where it persists is, as expected, in the upper half of the
regenerating matrix, the zone that is still immature and consolidating. In that zone, the bfgf occurs in the
developing mesh and some of the cells, but little is left at this time. Bfgf clearly has a strong correlation with
active fibrogenesis, and peak bfgf expression correlates with the peak of
fibrogenic activity. However, the
views at 17 and 30 days call into question whether bfgf is an inducer or
governor of the process versus just a passive or secondary element that marks
but does not necessarily regulate the process. These variances from expectation call into
question our knowledge and understanding about the role of bfgf in wounds and
tissue biology. |
||||
|
|
|
|||
|
This and the next few slides are a recapitulation of the key
events in Integra histogenesis. When
it is applied to a properly prepared wound,
there is never an inflammatory or defensive response. It is just the opposite, inflammation is
suppressed. Note that Integra
chemistry is very stable. Biopsies of
tissues up to four years later show preservation of much of the original
matrix. It is apparently not degraded
by cellular or enzymatic or other active means. Instead, it appears to degrade slowly by
simple passive hydrolysis. What this
means is that Integra has no influence on the host by any sort of signaling
or diffusion of chemical components.
Host cells find the matrix by randomness or happenstance and then
recognize the chemicals or surface properties of the material by direct
contact. Furthermore, because platelet
binding sites on the collagen are masked, platelets cannot “see” the
matrix. This means that when Integra suppresses inflammation, is not via an
active process or signaling or suppression of leukocytes. Instead, it more properly prevents inflammation by taking away
any recognition of a state that triggers inflammation. The matrix sits on the wound undisturbed
until enough pioneer cells find the material and initiate the regeneration. |
||||
|
|
|
|||
|
The cells that find the matrix and respond appear to be a type
of stem cell, presumably blood borne and ultimately from bone marrow. They find the matrix by random encounter
while these cells are “on patrol”.
Since pure collagen products do attract or bind such cells, another presumption
is that the glycosaminoglycans in the material are the recognition agent, a
hypothesis consistent with general principles of embryonic histogenesis. Left, a small
lymphoid looking pioneer cell has found the matrix and attached itself. Right,
two similar cells have transitioned to the next stage, a commitment to a
specific cell type, specifically, the dermatoblast, an embryonic type of
fibroblast. These transitional cells are spreading
out and binding to the matrix over a larger surface. They are also accumulating nucleoplasm and
cytoplasm in preparation for increased metabolic activity, including secretory
proteogenesis (collagen, etc.) and mitosis. |
||||
|
|
|
|||
|
As the transitional cells complete their transformation to dermatoblast,
they take on distinctive features. The
cells are large, with an enlarged nucleus and even larger cytoplasm. The cytoplasm seems granular, reticulated,
or textured, and the borders at times seem indistinct, giving the cells a
“fuzzy” appearance. When these cells
mitose and multiply, they create small clusters of similar cells. Since the intercellular boundaries cannot
be easily seen, they appear as a syncytium, hence the term syncytial
fibroblasts and syncytial clusters. “Syncytial fibroblast” is descriptive of
their appearance, whereas “dermatoblast” describes their role. Left, Integra at that
stage where transitional cells have transformed to large individual
dermatoblasts. In looking at the
specimens of many patients, it is interesting that the morphology of these
cells and the overall process have distinctive personal differences or recognizable
variations. It is like looking at
faces or animals in a herd – they are all people (or whatever species), and
they are all the same in the most important ways, yet individuals can readily
be recognized. In this specimen, this
subjects dermatoblasts have an elongated wispy or spindle shape, where as in
other patients they are rounder or polygonal, yet all have the basic
attributes of lacy large cytoplasm. Right, in the upper center of the
image is a syncytial cluster, a group of dermatoblasts that are the progeny
of an original pioneer cell that transitioned, transformed, then began
mitosis. This cluster may have about
7-12 cells (accounting for unseen 3-dimensional geometry). It is already functioning to produce young
fibrillar collagen, but it is limited in capacity for further mitosis and
fibrogenesis because it does not yet have a blood supply. In principle, this cluster should now be
making significant amounts of vegf, and that is confirmed by the immuno
stains shown on a prior panel. The effect
of the vegf is to stimulate the closest nearby vessels, those underneath in
the host wound. Indeed, at bottom
center can be seen migratory cells moving into the matrix from the host. Text,
references a research paper that describes embryonic dermatogenesis,
including a clear description of the embryonic dermatoblast, “ . . . a watery,
cellular network of mesenchyme that is joined through long slender
pseudopodia processes and specialized intercellular junctions into a
syncytium . . . ”. Holbrook KA, Smith LT: Ultrastructural aspects of human skin
during the embryonic, fetal, premature, neonatal, and adult periods of
life. Birth Defects 17: 9-38, 1981. |
||||
|
|
|
|||
|
Once the syncytial clusters attract blood vessels and blood
supply, they can then begin a second set of mitosis and fibroplasia, they
form that will become mature solid dermal structure. The progression from isolated stem cells
and their metamorphic forms to active space filling histogenesis can be seen
here. Left, a wide view of
Integra on a wound. There is not yet a
physical connection of matrix to host, but the matrix is well populated by
individual cells. The various stages
of this afferent histogenesis can be seen:
small lymphoid pioneer cells, flat matrix-adherent transitional cells,
and then the large metamorphic syncytial cells, some in pairs or small
clusters. Absent a blood supply, these
cells cannot create more cells or substance, as those that are there already
are competing for and consuming the available supply of oxygen. Right,
visible here is the response to the circumstances on the left, and the
transition to the second set or efferent phase of histogenesis. Notice the hypertrophy, hyperplasia, and
basophilia of vessels and angioid cells in the host tissue subjacent to the
matrix. Streaming or entrainment of
cells migrating from existing vessels below to the chemotactic source above
is evident. At the base layer of the
Integra matrix there are more cells within individual clusters, and there is
more collagen filling the pores in the matrix. There is a hint that physical connection of
the matrix to the host is about to occur as this second set fibroplasia
evolves. |
||||
|
|
|
|||
|
This view shows more advanced proliferation of vessels from the
host and ingrowth into the developing matrix.
Left, vessel sits at the
boundary between host and Integra, the first to intercept vegf that is
diffusing from syncytial fibroblasts above.
Hypertrophy, proliferation, and migration of angiocytes is present,
and there is a clear sense that stimulated ells are pushing up in to the
matrix. The response of this vessel to
stimulus is intense enough that the normal luminal architecture it once had
is obliterated. It is a visual treat
to see but not so surprising that the hyperplasia and response are occurring
on the windward but not the leeward side of the vegf stream. Right,
a similar event, in this case with diffuse angiohypertrophy along the length
of the donor vessel. As expected, the
matrix is populated by syncytial clusters without yet any second set
fibroplasia, density, or consolidation.
The syncytial cells and clusters are relatively large and numerous,
perhaps explaining an intensity of stimulus that would have provoked the
donor vessel to respond so strongly. In both images, the vessel response is more of an intact or
coherent sprout branching from the donor for short distances into the matrix
rather than individual angiocytes migrating long distances as seen in normal
inflammatory wound healing. Coherent
sprouts over short distances is very typical of regulated embryonic
angiogenesis and entirely unlike what happens during normal wound healing. The dynamics of angiogenesis in these structures is another
opportunity to compare and contrast matrix regeneration versus wound
healing. During normal wound repair,
angiocytes and vessels are the first restorative or histogenetic cells to
appear in the wound (summoned by macrophages). Fibroblasts appear after that. In Integra, non-vascular histogenetic cells
appear first, the dermatoblasts. These
cells proliferate into small clusters which, just like in embryonic
vasculogenesis, this small locus of cells can become only so large until new
blood supply is attracted. This is how
blood vessels and the vasculature develop during normal embryogenesis. It is also what is happening during Integra
histogenesis. The fibroblast cells
appear first, then they attract vascular cells, and then when vessels arrive
(often as short well formed sprouts), the hypoxia is relieved, the vegf turns
off, and no more new sprouting or angiohyperplasia takes place. This closed-loop regulated process creates
a vascular network embedded in the new tissue that has just the correct
vascular density to supply the precise needs of the tissue. In contrast, in normal wound healing, the
first-to-appear angiocytes just keep coming until inflammation and its
angiogenic cytokines decide to turn off.
This open-loop process means that vessel count builds to inordinate
densities and vessel lengths, thus the appearance of red granulation tissue
(versus white dermis or neodermis). |
||||
|
|
|
|||
|
Vascularization can only come in from established vessels below,
and thus second set consolidative fibroplasia must start at the bottom
adjacent to the host. Once vessels are
established at the basal layer, they can then source cells and vessels to the
space above them, and then second set fibroplasia can begin in there as well. This process keeps rising as vessels grow
ever higher toward the outer surface of the matrix. As this system progresses, Integra matrix
becomes progressively filled with the new living dermal analogue. Left, right lower
corner is the original wound. Working
up and to the left, the sequence of regenerative events is revealed. At the host-matrix interface, there is now
a solid adhesion of new dermis to the body.
Vascular ingrowth is obvious, and surrounding the new vascular
pedicles there is fibroplasia with new cells and new collagen. Higher up, in the left upper zone,
syncytial clusters are awaiting the arrival of new vessels so that they can
begin the efferent fibroplasia. Right, the same process, but it is
now more advanced, and the matrix is mostly filled. While the histogenetic process must rise
sequentially through the matrix from top to bottom due to the finite rate at
which new vessels can extend themselves, nonetheless the qualitative features
of the process are the same in all domains at all levels. Thus, when the process is complete, the
entire matrix has a uniform architecture with no overall spatial orientation
or segmented or polarized architecture. These views show the matrix in its most active phase of
histogenesis. The matrix and its regeneration are a
mesenchymal process, involving cells and tissues from the embryonic mesoderm. It is also a purely mesenchymal process,
independent of epithelium. (Even
though skin grafts can be added later, the matrix by itself does not host or
engender epithelial or adenoid proliferation from skin, skin appendages, or
other epithelia.) The second set
fibrogenesis illustrated here is another aspect of Integra histogenesis that
is different than inflammatory wound healing.
In normal wound healing, angiocytes and vessels arrive first, then
behind them come the fibroblasts to make dense collagen scar. In Integra histogenesis, the fibrous cells
come first. Revascularization is
necessary for the early clusters to proceed with more robust and orderly fibrogenesis,
but the fibrous cells are already in place.
Unlike normal post-inflammatory wound healing which regenerates a scar
and generic stroma, the Integra matrix makes a dermal analogue, including the
specialized syncytial fibroblast which is equivalent to the embryonic
dermatoblast. Although these two
systems are built of just the same two cells (angiocytes and fibroblasts) and
their respective outputs (vessels and connectives), the morphology and fine
details of the structures they build are significantly different. |
||||
|
|
|
|||
|
Once the histogenic process is complete and the matrix is
uniformly filled, the mesenchymal or stromal part of the regeneration is
complete. The resulting new living
tissue is a high quality dermal analogue, having cell morphology, cell and
tissue level anatomy, organizational dynamics, and mechanical properties that
are a close match to normal skin and entirely different than scar. However, the complete transformation to new
skin is not quite complete yet.
Epithelium is needed, and that is supplied either by natural
epithelial migration and ingrowth from the wound margins (a clinically
suitable strategy for small wounds) or else by skin grafting. Once the skin grafts are applied, the
transition to a high grade new skin is rapid. Left, the Integra
matrix when it is fully regenerated and capable of supporting a skin graft. The structure and its morphology are
governed in part by the geometry of the original matrix, but the structure
otherwise shares many of the quintessential features of proper embryonically
generated dermis, not scar. Second, early after a skin
graft. It is firmly adherent to the
matrix, but it is still thin, just starting the process of reestablishing
itself as a functioning epithelium. The
basal layer is still somewhat disordered, still trying to reorganize into a
correct stratum germinativum. Note
that the graft is in direct contact and adherence to the regenerated Integra
matrix. The matrix and neodermis look
as they should, no change in structure or regeneration status, and no new
mesenchymal elements that were not there when the graft was placed. Third,
the graft is now well established and starting to reconstruct its own normal
anatomy. A normal layer of basal cells
has reformed, and they are generating cells properly as seen by the increase
in acanthocytes. Furthermore, dermal
papillae and rete ridges are starting to form, structures and a geometry necessary
to increase surface area and minimize diffusion pathways so that gas and
substrate supply are maintained to the more massive epidermis. As dermal papillation occurs, a new layer
of mesenchyme is starting to develop between epidermis and the Integra
neodermis. It is still thin here, but
there clearly is a separation between epidermis and Integra neodermis due to
the formation of this new cellular and collagenous zone. This is a classic lamina propria for the
epidermis. Furthermore,
angiohypertrophy is seen near the top of the Integra because these older
vessels now become the source of the new subepidermal plexus and papillary
tufts that nourish the epidermis. Right, mature Integra skin 1 year
later. The epidermis is mature in all respects, including mature papillation
with rete ridges. A normal papillary (subepidermal)
dermis has fully formed. It contains mature
subepidermal and papillary blood vessels as are expected to be there in
normal skin. The original Integra
sponge is still present below, perhaps thinning out here and there, but still
having an overall normal appearance, without evidence of contraction. Gross architecture of the regenerated
Integra, which is the now the reticular dermis, is quite similar to normal
dermis in terms of the organization and density of collagen fibers. Note what has happened through this process. The composite new skin has a proper
architecture and mechanics. Epidermis
has restored itself. In so doing it
generated its own lamina propria by signaling to the structure below. This is analogous to the embryonic
state. Integra histogenesis and
embryonic dermatogenesis are the same thing.
They make the primary dermis, the reticular dermis. Reticular dermis is a primary structure. Integra is a primary structure. Papillary dermis is a secondary induced
structure, called into existence by the epidermis. Laminae propria are present as a service
layer under all epithelia. For many
organs, e.g. bowel and bronchi, the laminae propria are easily distinguished
from the primary structures because they are different, connective tissue
versus smooth muscle or cartilage or whatever. In the skin however, the lamina propria has
the same elements and general appearance as the primary tissue, so the two
are always discussed as one unit structure, but embryologically they are
not. Furthermore, we can now
appreciate the versatility of fibroblasts and angiocytes, or their discipline
to respond to whatever commands are issued, to respond to different
circumstances and inputs with different patterns and structures. In that sense they are like any
construction material – they will make what you want if you give them the
right instructions and assemble them in the right order. These same two cells and their derivative
vessels and connectives can (1) make scar in response to inflammation, or (2)
make primary reticular dermis in response to an aminoglycan stem cell
aggregator indicating embryonic conditions, and (3) make papillary dermis in
response to an epithelium needing to restore its lamina propria. |
||||
|
|
|
|||
|
Here is another “time lapse” composite view of the Integra
histogenetic process. Left upper, at 5five days, there is
no physical connection between wound and Integra. The matrix has lightly scattered cells,
mostly still pioneer or transitional cells, but some syncytial clusters have
formed, active dermatoblasts that need a blood supply. Angiohyperplasia is evident in the host,
and angioid cells are seen streaming through tissue to enter the matrix. Right
upper, at 10 days, angiohypertrophy and hyperplasia are substantial. Streams of entrained cells are moving large
numbers of cells into the matrix. The
upper half of the matrix is no different than the 5 day view, with pioneer
cells and a few syncytial cells.
Syncytial clusters can be seen at mid matrix. In the lower half of the matrix, early
clusters have given way to domain-filling cellular proliferation. Proliferation is most dense closest to
hypertrophied source vessels, where vasculogenesis into the matrix first
occurs. An actual physical or
anatomical connection of matrix to host is just beginning in those areas of
vascular infiltration. Left lower, at 18 days, the process
is advanced. Well organized and
clearly delineated blood vessels have entered the matrix, and the lower
layers have dense filling with cells and collagen. Thus there is now a firm fibrous connection
of matrix to host. Matrix-filling
histogenesis is now active in the mid and upper layers. Empty matrix with only syncytial clusters
are still present only in the thin topmost stratum. Angiohyperplasia is still present, but it
is beginning to wane or involute in the host.
Active angiohyperplasia has transferred to the mid level of the matrix
where new vessels are now the source for the current histogenesis taking
place in the upper layers. Right lower, maturing regeneration at
54 days. Although there is fixation
artifact creating false empty space between tissue domains and matrix septae,
the matrix is mostly filled with tissue (some domains still empty at the
top). The regenerated tissue is
largely eosinophilic due to collagen, without the intense basophilia due to
dense cellularity. Angiohyperplasia or
its residue are not fully abated but nearly so, and host or substrate anatomy
is returning to normal. Collagen
binding of substrate to new tissue in the matrix is advanced, and mature
vessels bridging the interface are obvious.
Note that the lower parts of the matrix are thicker, more expanded
from more collagen, whereas the upper strata are flatter where there is less
collagen. This confirms that the
matrix does expand vertically, filling out the pores more fully, getting
thicker and more voluminous with progressive histogenesis and the
consolidation of domains. |
||||
|
|
|
|||
|
Here is yet another “time lapse” composite of Integra
histogenesis, a vertical rather than a horizontal view, giving a better sense
of the timewise development of the new tissue. Note that “## days” (after placement of the
Integra) simply documents when each specimen was taken, and is not to be
interpreted as being a strict timescale of histogenesis and
regeneration. The times shown do
accurately reflect the general process and times that occur, but there are
variances from patient to patient, time to time, place to place, and even one
millimeter to the next in any specimen.
The “17 days” specimens from two different patients show two close but
different phases of the regeneration process.
At 5 days, the matrix is
empty of proteins, glycans, and any other formed substance. Early pioneer cells sparsely populate the
matrix, independent of distance from the host. There is no physical connection of matrix
to host. Some of the cells are
adhering to the matrix, entering their transitional phase before becoming
actively proteogenic, mitotic,
histogenetic syncytial fibroblasts. At
13 days, cells have transformed
into syncytial fibroblasts, and clusters of such cells are present. There is still no other substance or
biochemical matrix. These clusters are
capable of functioning with the oxygen and nutrients that diffuse from subjacent
host vessels, but they are reaching their limits of growth and activity until
direct vascularization of the matrix occurs.
At 17 days, left, the matrix is populated by
large syncytial fibroblasts. At the
interface with the host wound, angiohyperplasia is evident, and migration and
ingrowth of cells from host vessel into matrix can be seen. Surrounding this zone of vascular
infiltration, cell density is increasing in the sponge, and early organized
collagen is appearing. At 17 days, right, the process of vascular infiltration and progressive
histogenesis is highly active. There
is a firm physical connection between host and Integra. angiohypertrophy is still evident at the
base, and new vessels are reaching far into the matrix. At the lowest levels, eosinophilic pink
collagen deposition is dense. In the
upper half, just above the large vessels, is a basophilic zone of small
capillaries supporting dense proliferating fibroblasts which are just
starting to make collagen. The
non-staining upper stratum has pioneer and syncytial cells in otherwise still
empty domains awaiting the arrival of new vessels. At 30
days, the process continues, progressing to the top of the matrix. Vascular ingrowth is now evident throughout
the matrix, with larger conducting vessels rising high enough to permit
substrate supply to the top stratum.
Basal angiohyperplasia has subsided because histoprogenitor cells at
this level no longer feel the effects of proliferative cytokines coming from
the now far away active histogenesis zone.
Lower strata of the regenerated matrix are increasingly eosinophilic
as collagen accumulates and matures, and fibrocytes become thinner and less
active. In the upper half, there is
still a purple basophilic balance to the color, due to a higher density of
cellular cytoplasm and nucleoplasm, and a relative lack of collagen. This zone corresponds to what was starting
in the basal area in 17-left, and what was occurring above the middle in
17-right. At 42 days, the entire matrix is now filled with collagen. Cell proliferation in the host is subsided,
and cell prominence throughout the matrix has lessened as vascular and
fibrous cells retreat into smaller flatter mature forms. Vascular density is uniform throughout the
regenerated matrix. However, note that
there are still differences between the upper and lower strata. Below, collagen is pinker, denser, more
organized, whereas above, there is still a relative basophilia, , and
collagen is less dense or organized.
At 70 days, the process is
now almost uniformly complete throughout the matrix, with only a slight
residual basophilic tint in the topmost zone.
New vessels crossing the original interface and the tissues of the
host have returned to completely normal appearance and cell density. Note how the matrix gets progressively
thicker as collagen fills up the sponge domains. Papillomatosis of the Integra never occurs,
meaning that the material is not expanding tangentially, only vertically, and
not creating new tissue in excess outside the confines of the scaffold. |
||||
|
|
|
|||
|
Integra histogenesis, the process and the resulting anatomy, are
discernible by studying the histology of the events. The process is quite consistent, virtually
no variation in the observed events, sequences, operational dynamics from one
patient to the next. It is distinctly
different than normal inflammatory wound healing and scar, and it is remarkably
similar to embryonic processes of vasculogenesis and dermatogenesis. One of the most important characteristics of Integra, and one of
the biggest distinctions between it and normal wound healing, concerns its physics
or system level operational dynamics.
Unlike wound healing’s “overshoot then involute” process of open loop
unregulated excessive formation of scar, Integra histogenesis is a process of
steadily building up a mature tissue, beginning with nothing, and
asymptotically approaching the final correct model. Integra histogenesis has the same sort of
feedbacks and closed loop controls that characterize all of embryology,
without which an embryo could never develop properly. These controls, in embryogenesis in general
but also in Integra histogenesis, steadily build toward the reference anatomy. Unlike wound healing which is rapid, a
wound typically “healed” in 1-2 weeks, matrix histogenesis requires more
time, typically 4 weeks for the Integra matrix to fill from base to ceiling. However, once the domains consolidate and
mature, typically within 2-3 months, often less time, the process is
essentially complete, unlike the many months or years required for wound
healing scar to subside and involute to tissue with normal properties. Left, a long vertical
image to remind of the overall appearance of a fully complete Integra
reconstruction many months later.
Matrix persists as part of a primary reticular neodermis that looks
and functions much like normal dermis.
Above that is a secondary papillary dermis forced into existence by
the overlying skin graft as its lamina propria. Above that is the mature epidermis. Below it all is the original wound or
native host. Center, a reminder of the sequence of events, first pioneer and
transitional cells, then syncytial fibroblast transformation and clusters
(the embryonic dermatoblast), then angiogenesis and revascularization, then domain-filling
second set fibrogenesis which rises through the matrix in synchrony with the
rising neovasculature. Once the matrix
is filled and consolidated, it is much like normal dermis histologically and
mechanically. Right, two views showing the matrix at mid regeneration circa 2 1/2 weeks and mature
at 10 weeks. Zones of primary
histogenesis (pioneer and syncytial cells), fibroplasia (early collagen deposition
and domain filling), and consolidation (maturation) are marked. Graph: Just as we mapped it for inflammatory wound
healing, this shows the status of Integra regeneration via a vague
nondescript measure of quality and quantity of wound elements and
organization versus time after injury.
The dotted line is a target level representing the quality and
characteristics of normal skin or stromal tissues. The graph shows the behavior of the
histogenic process, beginning at the beginning with not much “stuff”. What Integra and other regenerative
matrices do is to execute their activities in a regulated measured way
following the same closed-loop reference-based controls that govern embryonic
angiogenesis. Another key distinction
between wound healing and matrix histogenesis is the spatial dynamics of the
process. Wound healing is a gradient
field process. The stimulus is in a
broad plane in the ceiling above the process, and responder cells are
garrisoned in the host in the basement, and the process is one of the
responder army marching ever upward through the structure, resulting in the
stratified and radiating anatomy that is so typical of granulation
tissue. In contrast, Integra
histogenesis is distributed evenly throughout the matrix, just like normal
embryogenesis. Recall that when
pioneer cells come, they occupy evenly the entire matrix from bottom to
top. Each becomes a locus of local
histogenesis, and all loci are equivalent.
It is true that the second set histogenesis follows a rising wave from
low to high, but that is just a time delay due to the rate at which new
vessels can reach the farthest loci, but once vessels arrive, each locus
behaves as any other, up, down, this side, that side, earlier, later – all
domains are the same, and the regenerated material is homogeneous. The process deposits cells and vessels and
connective materials in relationships and densities that match those found in
normal (embryonically generated) tissues.
That is why Integra regenerated tissues have characteristics so
similar to native tissues and so different than scar. Once the initial regeneration is complete,
within just several weeks, there is no need to remodel the new tissue as it
is already so similar to normal dermis. |
||||
|
|
|
|||
|
Integra
Regeneration Correlation with Primary Developmental
Histogenesis – Variances from Ordinary Inflammatory-Fibrous Wound
Healing - Embryonic Tissue Formation. The distinction between Integra histogenesis and inflammatory
wound healing has been central in this discussion. Furthermore, many of the panels and
paragraphs already presented have discussed in greater or lesser detail the
similarity of the Integra regeneration process to normal embryogenic
processes. This section will provide
additional correlation of those hypotheses. This is another
illustration from Pietro da Cortona, Tabula XVII, a view of the back and
spinal nerves. It reminds that there
is much to learn by looking under the surface of casual appearances and
studying a subject with discrimination and detail. |
||||
|
|
|
|||
|
These images were reviewed on prior panels. The top image shows active wound healing
and active Integra histogenesis side-by-side on the same specimen. The bottom image shows old scar and old
Integra likewise side-by-side. The
sheer visual contrast is enough to persuade that these processes are
different, that although both invoke only angiocytes and fibroblasts, that they
are orchestrating the response in entirely different ways. In the music of mesenchymal histogenesis,
understanding clearly why the same few notes, angiocytes and fibroblasts,
vessels and connectives, can be scored so differently by different composers
or conductors, injury-inflammation versus matrix-embryogenesis, is central to
understanding how we can use these properties to clinical and therapeutic
advantage. To reiterate the metaphor
of this presentation, wound healing is classic, traditional, the biological
Old World, the underpinnings of our surgical civilization, whereas matrix
regeneration is the recently discovered biological Healing wounds
and growing tissues is comparable to any agricultural endeavor, and like
successful crops, successful wound closures are of vital concern to
individuals and the community as a whole.
Left, statue of Persephone. In Greek mythology, Persephone (Περσεφόνη), was the daughter of Zeus and the harvest goddess
Demeter. Abducted by Hades to the
underworld, her yearly return to the surface to visit her mother is the
mythical reason for the spring growing season then the autumn and harvest before
winter returns. This Roman statue is from the beginning of 1st century CE,
from the |
||||
|
|
|
|||
|
This panel compares key elements and events between natural
wound healing (Wound) and Integra histogenesis (Integra). Arrival & recognition.
For any reactive biological process to be initiated, something has to
recognize or be triggered by a variance or perturbation of the system. Something has to first “see” the injury or
wound to start the response that protects then repairs things. Wound. Blood borne platelets are first to recognize
an injury or wound via recognition of certain extra-vascular chemicals and
products of thrombosis. Blood borne
leukocytes are the first to populate the wound, for the sake of acute host
defense and control of the injury, marshaled by platelets and thrombosis via
a combination of diffusible chemotactic factors and in situ coagulum based
chemistry. Integra. The injury is masked from recognition so
the inflammation-defense system does not see the wound. Matrix is recognized by pioneer cells that
seem to be tissue stem cells. They are
presumably marrow derived cells. While
blood would have to be the means of transport for these cells, they are not
plasma-resident cells the way platelets and leukocytes are. They are probably cells that patrol tissues
and find the matrix by happenstance.
Active recruitment or attraction to the matrix is highly unlikely
since they arrive only days later after all vestiges of acute phase injury
and response have lapsed, and because Integra does not appear to source any
soluble or diffusible factors.
Recognition of the matrix is almost certainly a function of direct
contact of the cells with the aminoglycans in the Integra sponge, since that
is consistent with what is known about ordinary and embryonic histogenesis,
and because such cells are not seen with collagen-only matrices. Stimulus & activation.
Once the new event or condition has been recognized, then something
needs to stimulate or activate the response.
Wound. Activation
in a wound is due to chemotaxis-chemotropism between the inflammatory cells
that were called to the wound and latent histoprogenitor cells resident in
the host tissues below the wound. Platelet
derived pdgf or similar transforming cytokines convert blood borne monocytes
to tissue macrophages. Among their
several functions, they are the chemotaxis agents, issuing their own growth
factors which signal the tissue histoprogenitor cells. Activated progenitor cells first become
angiocytes, chemotropic cells which follow the angiogenic signals and migrate
toward the surface of the wound where the macrophages are. Integra. Activation with Integra is on the matrix,
an interaction between the pioneer histoprogenitor cells which found the
matrix and the matrix itself. This
process is initiated by direct surface-to-surface contact interaction between
the two elements. Once the recognition
occurs, activation causes the cells to bind to the surface. This is followed by accumulation of
nucleoplasm and cytoplasm in order to become phenotypically differentiated,
committed to a certain cell type, and functionally active. Mitosis and species specific functions then
begin. In this case the cell type is
the dermatoblast, and the function is the production of a connective protein
mesh. Organization. Once the
proliferative or histogenic process begins, cells that respond and the
chemicals they make must organize into a functioning anatomy. There is a level of biological organization
that comes between “cell” and “tissue”, call it “cellular assemblies”. The initial assemblies in wounds and
Integra are different. Wound. The first structures in the wound are
vascular cords. These are the
reassembly of migrating angiocytes as they stream from source vessels in the
sub-wound toward chemotactic signals in the top layers. These new vessels are long linear channels
that are unlikely to branch along the way.
This morphology, typical of a gradient field, is unlike embryonic
angiogenesis where vessel formation occurs in distributed fields and is
highly meshed, branched, or collateralized.
Integra. The first
structures in the Integra matrix are syncytial clusters, assemblies of
several embryonic dermatoblasts which are all mitotic daughters of the
initial pioneer-transitional cells.
Unlike initial wound vessels which are a reassembly of migratory
individuals, syncytial clusters arise from a one-cell anlage, comparable to
tissue and structure formation during embryonic growth and development. Early structure.
Early structure means the assemblage of assemblies, and the intermix
of multiple elements into a defined tissue.
Wound. Following
the migration and assembly of vascular cells and vessels, fibroblasts then
appear. They begin to make connective
matrix, and the vascular and fibrous elements become intermixed. This new biomaterial will go through subsequent
phases of consolidation then maturation, but at this point, all of the
constituent elements are there. Integra. At this point, syncytial clusters have
grown as large as the absence of blood supply permits, so they make
angiogenic factors to attract vessels.
Vessels arise, and then second set histogenesis can begin. That establishes foci of the new tissue, an
intermix of new vessels with old and new fibroblasts (the original
dermatoblasts then second set fibroblasts).
In wound healing, the events occur in sequential tangential strata but
they occur simultaneously across the entire spatial surface. In Integra, a fully organized tissue
develops rather quickly within each individual small focus (site of a syncytial
cluster or within a pore of the sponge), but the filling and coalescence of
all domains takes time, a distributed model of histogenesis comparable to
embryogenesis. Advanced structure.
Early structure must coalesce or condense into a structure that is the
nominal output or end point of the acute process and which serves the host
for the sake of biological safety and functional adequacy. After that, the resulting tissue can
remodel if necessary back to a state comparable to original normal anatomy. Wound. The culmination of vascular and fibrous
proliferation is a scar, a dense amalgamation of thick collagen bundles that
pack all space. The bundles are
randomly oriented, and absent any clefts or spaces, the material is very
inelastic and undeformable. It is
strong, meant to cement the body back together, but it is prone to
contractures and impaired motion. late
maturation back to normal tissue takes many months or years. Integra. The culmination of the Integra process is
that all of the minute histogenic foci eventually fill all pores in the
matrix. The tissue is not structurally
strong since the individual “pearls” of new tissue do not have large
interconnections, but the tissue has pores and spaces and septations from the
Integra sponge which preserve the pliability and elasticity of a normal
(embryonically developed) dermis. Late
maturation remodels the material to a histologic appearance comparable to
native dermis, but the physical and biological properties of the material are
not much changed during that late maturation since they were close to normal
to begin with. |
||||
|
|
|
|||
|
This panel further compares natural wound healing versus Integra
histogenesis, specifically angiogenesis and blood vessel formation. At its simplest, the angiogenesis of normal
inflammatory wound healing is an open loop unregulated process that results
in extraordinary overproduction of blood vessels and high vascular density in
the active wound and the final scar.
Integra angiogenesis occurs under the same regulated controls as
normal embryonic angiogenesis, resulting in a vascular density that is just
what it should be for the cell count and metabolic needs of the tissue. Left column, angiogenesis in normal inflammatory wound
healing. Under the microscope,
angiogenesis is seen as dense, closely distributed large vessels carrying
large volumes of blood. High vessel
count and density plus high vascular and blood volumes mean that
volume-per-volume the wound and granulation tissue are dense with blood. High blood density is seen grossly as the
exuberant congestion and red color of granulation tissue. Right column, Integra angiogenesis. (Wound and Integra microscopic views are
presented at same magnification.) In
Integra, the microscopic view shows that vessel density is low. It is comparable to normal dermis and
fascias. Vessel caliber is small and
more uniform, in the capillary and pre- and post-capillary sizes. Grossly, the lower density of vessels and
blood means that the regenerated tissue is pale. It
is like any other normal fibrous tissue such as dermis and muscular fascias,
white or only marginally pink, because blood volume in these tissues is
slight. (The regenerated material is sufficiently thin to be slightly translucent,
so the gross color appears in part like whatever is behind the thin material,
lumbar muscles in the center photo, achilles tendon in the lower photo.) Inflammatory wound healing.
Granulation tissue is dense with excessive blood vessels. The reason is that inflammatory
angiogenesis is an unregulated open loop process forced by an agent extrinsic
to the new tissue forming process. The
key dynamic is an interaction between stimulatory macrophages and responder
angiocytes. Macrophages make angiogenic
factors to attract angiocytes.
However, blood borne macrophages are not part of the original tissue nor
the final tissue (only angiocytes and fibroblasts are). Thus they are extrinsic to the
tissue-stroma-scar, and they will clear out as the final scar evolves. The vessels they attract are not for
themselves, and they have no way nor even a need to regulate the degree of
angiocyte response. If macrophage
stimulated angiogenesis was a regulated or closed loop process, then arrival
of angiocytes or vessels or blood flow would turn off the chemotaxis. However, that is not the case. The process is open loop and unregulated
meaning that revascularization does not suppress the macrophages. Regardless that vessels arrive, macrophages
keep making chemotactic angiogenic factors, keep attracting new vessels, and
vessels keep coming until acute inflammation winds down and macrophages and
inflammatory cells disappear. The
proliferation of new blood vessels is thus very dense, an overabundance far
in excess of what is needed by normal healthy dermis or fascias. This is seen histologically as an excessive
“unnatural” number of unusually large blood vessels. Excess blood volume in the excessive
network is seen grossly as bright red “granulation tissue”, the clinical
signature of inflammatory wound healing. Integra histogenesis.
In contrast, Integra histogenesis results in a controlled angiogenesis
in which there is feedback between burgeoning histogenic cells and the
vessels that they attract. This system
of closed loop control is exactly the same as the system that governs
efficient embryonic vasculogenesis. As
seen in the images, blood vessels are present as in any living tissue, but
their numbers and density are very low compared to what is seen in
inflammatory granulation tissue. Just
like normal tissues, vascular density in the Integra is precisely what it
should be for the cell and metabolic load of the tissue. This is due to the dynamics of the
process. This is a regulated closed
loop process. Vessels do not respond
until summoned, in this case by the new dermatoblasts. As new vessels arrive, ischemia of the
clusters is relieved, and they stop making vegf, so vascular stimulation and
taxis ceases. It means that vessel
count and vascular density are precisely what they must be to supply the developmental
and metabolic needs of the new tissue, neither more nor less. This is identical to the process of
embryonic angiogenesis and the regulation of vascular density in normal
tissues. Normal dermis and fascias
appear white because they have relatively low metabolic requirements and thus
a low vascular density. However, they
are living tissues, so they are getting the correct blood flow. They have precisely the number of vessels
that they need to function and be alive.
Regenerated Integra, which is structurally and functionally similar to
these tissues, looks the same as them, which is quite distinct from densely
vascularized wound granulation tissue. Embryonic histogenesis.
Embryonic histogenesis is not explicitly illustrated, but the process
is understood. Embryonic angiogenesis
must be understood as a system via the physics of non-linear dynamics. The VT (Vascular neT) model of angiogenesis
explains the process. What happens in
embryonic growth is that individual locales of proliferating tissue trigger
tropic angiogenesis only as metabolic loads outstrip available supply. Arrival of blood vessels suppresses further
angiogenic stimulation, and vascular density ends up being only exactly what
it should be to meet metabolic load.
Much of this system is understood, for instance that various
angiogenic cytokines can be involved, but that vegf is the predominant one;
that all proliferating or growing embryonic tissues make vegf during active
tissue growth; that the vegf gene is directly turned on and off by oxygen or
its absence; that vascular morphology
and density are highly organized and efficient, exhibiting the property of
“locality” where vessel growth is governed independently by distributed
locales of active growth of the host tissue.
These efficient dynamics leading to a closed-loop regulated non-excess
vascular density are the same as vessel formation during Integra
histogenesis. |
||||
|
|
|
|||
|
This panel further compares natural wound healing versus Integra
histogenesis, specifically fibrogenesis and connective matrix formation. One process makes scar, the other makes
dermis. These two structures, although
both made of only angiocytes and fibroblasts, vessels and connectives, have
remarkably different histological structures, materials properties, and
implications for health and function.
Just as for angiogenesis and vascularization, wound healing makes
unregulated excess material, whereas Integra makes proper amounts and
architecture comparable to normal dermis. Top, wound healing.
The young scar is dense with cells and collagen. Collagen is either amorphous or in large
bundles. Spatial orientation of the
fibers and bundles is isotropic, uniformly or randomly distributed in all
directions without a dominant axis.
Cells are not just abundant but vary in size and shape. There is no interruption of the mass by
clefts or spaces. It is inelastic,
non-distensible, non-deformable. Bottom, Integra histogenesis.
The collagen matrix is more orderly.
It seems to flow concentrically following the contours of its local
domain as defined by the Integra septae.
Cells are not dense, and they are uniformly flattened and mature. Spaces and internal deformability are
obligatory since the Integra septae prevent inter-domain adhesions. This allows for degrees of elasticity or
distensibility that more closely matches normal dermis. Top,
marble statue the Farnese Atlas, in the National Archaeological Museum
of Naples, Italy (Atlante
Farnese, Museo Archeologico Nazionale di Napoli). It is a 2nd century CE Roman copy of a
Hellenistic original. It depicts Atlas,
the Titan sentenced by Zeus to hold up the sky, bearing his burden by holding
the celestial spheres. At 7 feet (2.1
meters) tall, it is the oldest extant statue of a Titan, the oldest known art
representing the celestial spheres, and the oldest surviving pictorial record
of northern constellations. Like many
statues in Italy, “Farnese” refers to Cardinal Alessandro Farnese who
acquired and exhibited many classical sculptures in the Villa Farnese in the
early 16th century. It reminds that
scar, generated by normal wound healing, is strong and essential to healthy
life, but it can also be an inexorable burden when it is ill behaved or in
excess. Bottom, another Hopi Kachina doll. Housed in the Brooklyn Museum, New York, it
was collected in the Hopi pueblos by a museum expedition in 1904, date of
origin presumed late 19th century. At
22 inches (56 cm) tall, the figure wearing the elaborate tablita is Pahlikmana
(or Poliman, Butterfly Maiden). This
character is a springtime and planting character who may have other personas
such as a Corn Maiden during certain dance ceremonies. Her dances are prayers for rain and a good
harvest. |
||||
|
|
|
|||
|
In this panel, the comparison of natural wound healing and
Integra histogenesis is not about specific events like vascularization or
fibrogenesis, rather about the dynamics of the whole process. Wound healing and scar
overshoot-then-involute. Integra
histogenesis progressively builds a correct tissue model. Given enough time, both processes gradually
remodel to something comparable to normal dermis. However, scar starts with many
characteristics that are undesirable and counterproductive, then it takes
many years if ever to return to acceptable mechanics and function. In comparison, Integra is much like normal
dermis from the beginning. Its
remodeling to a more normal architecture is of academic interest, but its
practical and functional attributes are comparable to normal skin from the
start. Left, after inflammatory wound healing, there is gradual remodeling
of scar to eliminate excess cells and materials until it eventually resembles
normal dermis or fascia. The kinetics
of scar maturation are slow as the material revises itself from a thick,
stiff, non-compliant, congested, hypertrophic material to nearly normal. The photos show young scar (left)
with dense cellular collagen and numerous enlarged vessels. In a fully mature scar (right),
vessels have all returned to normal size and density, and collagen bundles are
wavy and more compliant. The graph
shows the overshoot-then-involute dynamics of quickly building too much material,
then slowly thinning the scar back to the reference of normal dermis. Rapid over-attraction, over-production,
over-dense accumulation of cells and connective mesh occurs because acute proliferative
repair is open loop and auto-amplifying without regulated controls. The acute “overshoot” phase, which evolves
in days to weeks, is the basis for scar’s undesirable properties. Once the wound is epithelialized and closed,
the acute process subsides, then the scar matures. Maturation is a gradual asymptotic
self-modification back to a structure similar to normal dermis or fascia, a
process requiring months to years. Right, Integra also goes through a nominal maturation, slowly
looking more like normal dermis and fascia, likewise over months to
years. The difference between scar and
Integra is that maturation and the approach to a normal tissue occur from
opposite directions. The photos show
young Integra (left) with its domains recently regenerated, the
original empty matrix now consolidated.
Years later (right), collagen is more mature, wavy and
compliant, just like dermis and old scar.
However, with regard to porosity, collagen density, and cell count, it
is no different now than at the beginning, or stated conversely, newly formed
Integra has the properties of dermis to begin with. The graph shows there is no
overshoot, just a persistent regulated build to a relatively normal
tissue. The acute proliferative phase
of building the new tissue occurs over weeks to months (unlike days to weeks
for acute wound healing). Like scar,
the latter maturation to a more normal appearance continues for years, but the
gross clinical features of healed Integra (appearance and compliance) are
present within the first few months, and they change little after that. Unlike wound healing which quickly and
completely fills available space with material then whittles it away, Integra
histogenesis is a process of steadily building a proper tissue of proper
density, beginning with nothing then asymptotically approaching the final
model. Integra histogenesis has closed
loop regulated controls that steadily build toward the reference anatomy
without ever having an overshoot condition that must be remodeled. |
||||
|
|
|
|||
|
This and the next panel go a step beyond the last one, comparing
not just wound healing versus Integra, but having a third comparison to
embryonic histogenesis. There are no
histology pictures of embryonic events for comparison, but the biology and
developmental dynamics of embryogenesis as already well researched and
understood can be used as the basis for comparison. Left column = inflammatory
repair. Center column = Integra
histogenesis. Right column =
embryonic histogenesis. Top row compares the earliest cells in each process, the recognizers or
first responders, and the triggers or regulators of later activities. For wound healing, they are
inflammatory leukocytes. Neutrophils
recognize and defend, monocyte-macrophages induce subsequent proliferation. They are open loop triggers with no
feedback control by the stimulated cells upon the stimulus. Being only on the surface, they create a front
of stimulus, and the underlying response forms sequential serial planar strata. The inflammatory cells do not themselves
become part of the final tissue, the scar.
For Integra histogenesis, pioneer stem cells recognize
the matrix then initiate regeneration without any defensive component. The process is distributed throughout the
regenerative space as independent foci of activity operating in
parallel. The histogenic process is
closed loop, the stimulus or controllers being inhibited by arrival and
reorganization of stimulated cells.
The pioneer cell initiators of the process become part of the ultimate
tissue. For embryonic
histogenesis, early cells act as blasts, focal anlagen for generative
clusters of differentiated tissue.
Tissue and organ generation is thus highly distributed and
parallel. Correct tissue models such as
the normal embryonic vasculature depend on highly regulated tightly
controlled feedback loops. There is
nothing inflammatory or defensive.
Each embryonic anlage cell and its progeny become part of the ultimate
tissue. Second row compares the main induced histogenic cells in each
process. (Angiogenesis is essential to
histogenesis, and vessels are mandatory to support the fibrogenic cells. However, this panel does not compare
angiogenesis between the processes. In
brief, in wound healing, open loop unregulated vessels proliferate first then
fibroblasts follow. In both Integra
and embryonic histogenesis, primary histogenic fibroblasts appear first, then
highly regulated proper-density angiogenesis follows.) For wound healing, fibroplasia
is via classic reactive fibroblasts.
These relatively small compact cells are the first and only type of
fibrogenic cell in the process, and they come late, following behind new
blood vessels. They pack the space in
high densities, and they make thick dense fibrous collagen. For Integra histogenesis, the
initial fibroplastic cells are the large syncytial ones. These have a size and architecture entirely
different than wound-reactive secondary fibroblasts. They appear in relatively sparse individual foci, and until
they get vascularized, the clusters they make have just a few cells. They make a very fine fibrillar or
amorphous collagen. For embryonic
histogenesis, embryonic dermatoblasts have the same
architectural and collagen features of
the Integra syncytial fibroblasts. Third row compares the presence and role of glycosaminoglycans
in each process.. For wound
healing, the process starts with only an injured surface. As the system proliferates, it occupies and
defines a volume, but there is no pre-defined histogenic space. No space means there is no medium in which
histogenic cells can function. The
system must first create a medium in which angiocytes and fibroblasts can
make the secondary fibrous mesh and organized stroma. Glycosaminoglycans are the prime chemicals
made in order to create a primitive “ground substance”, the medium for
proliferative cells. Aminoglycans are thus
an output of the proliferative process.
For Integra histogenesis, the matrix defines an a priori
space in which the process can evolve.
The process stays confined to that predefined volume. The imposed architecture of the space
regulates growth and self-assembly of new cells. Glycosaminoglycans already engineered into
the matrix have the pivotal role, providing the crucial recognition and
regulation signals that activate pioneer cells to begin histogenesis A secondary gel ground substance eventually
forms, but it is not required to permit the migration and organization of new
cells. Aminoglycans are an input to
the histogenic process. For embryonic
histogenesis, aminoglycans serve as a priori medium, post hoc medium,
and histogenic process regulator. They
are recognized as having a central role in embryonic histogenesis. Their role is best understood by observing
what happens in a fetal wound.
Intrauterine fetal injury or wound does not trigger inflammation. The injury simply repairs itself by the
accumulation of new cells and GAGs and a resumption of tissue specific
histogenesis. Fourth row compares the collagen and connective mesh that
develops in each process. For wound
healing, the earliest collagen has a fibrous architecture with thick
longitudinal fibers. That morphology
persists throughout the process, leading to thick non-compliant scar
bundles. Collagen is dense and
excessive, without free space or mechanical compliance of the final tissue. The scar has myofibroblasts inducing
contraction, and it is subject to Wolf’s law effects causing hypertrophy and
tendinous metaplasia in response to extrinsic forces. For Integra histogenesis, early
collagen has a non-fibrous amorphous appearance. If the hypothesis is true that this is
comparable to the embryonic dermatoblast, then this early collagen has a fine
fibrillar architecture. Later “second
set” collagen has larger thicker collagen fibers, but even then the
architecture is not as dense, not excessive, maintaining porosity and
mechanical compliance. Fiber direction
is governed by the host space, not by randomness nor mechanical loads. Contraction is not observed, neither
histologically nor grossly as contractures responding to mechanical
loads. As such it retains its original
non-scar characteristics even across joints.
For embryonic histogenesis, collagen matrix is what it
is for normal growth and histogenesis. It makes normal dermis, fascias, tendons
ligaments, etc., with fiber and bundle sizes, densities, and orientations
that are “normal”. Scar and scar
collagen do not occur in embryogenesis, not even after intrauterine
injury. Fibrous contraction and contractures
do not occur in embryogenesis. Graph row compares the dynamics each process. For wound healing, “overshoot-then-involute”
first makes indiscriminate excess new tissue, after which the excess is
remodeled back to a dermal or fascia analogue over many months or years. For Integra histogenesis, the
process remains regulated, asymptotically building up the elements of the
proto-tissue until the final correct tissue is achieved without
excesses. The process consolidates to
the reference anatomy. For embryonic
histogenesis, the process and its output are the “reference
anatomy”. Embryogenesis is normal, so
by definition it creates the correct output.
It creates normal tissues by the regulated self-assembly of primitive
elements in correct proportions and relationships, never in excess. Even when the embryo is injured (“fetal
wound repair”), the inflammatory overshoot-then-involute process never
occurs, only the resumption of the normal evenly paced histogenesis that
creates normal tissues. |
||||
|
|
|
|||
|
This panel compares various other features of the three
situations, to further support the hypothesis and observations that Integra is
comparable to embryonic histogenesis, and both processes are unlike
inflammatory wound healing. The table
recapitulates much of what has been already stated, so item by item
commentary is not made. Note that the
comparisons involve different classes of parameters, some anatomical (e.g. cells,
chemicals, architecture), some physiological (e.g. cell responses), and some
dynamical (process regulation and control).
and FETAL WOUND REPAIR inflammation inflammation triggers the process inflammation
is suppressed no inflammation control
cells chemotactically summoned resident local mesenchyme locally
developing mesenchyme marrow-derived cells (marrow derived?) patrol cells control cell
initiation extrinsic direction intrinsic
direction intrinsic direction by summoning cells by arriving
cells by resident cells type of cell
response defensive non-defensive,
histogenetic non-defensive, histogenetic type of
healing wound module generative generative and regenerative inflammatory repair (embryonic) histogenesis histogenesis dynamical
control system open-loop closed-loop closed-loop controllers extrinsic to tissue controllers intrinsic to
tissue controllers intrinsic to tissue order of
histogenesis angiogenesis leads fibroblasts
lead dermatoblasts lead fibroblasts follow angiogenesis follows angiogenesis follows vasculogenic
dynamics target or gradient angiogenesis distributed field angiogenesis distributed
field angiogenesis vascular
density hyperdensity angiogenesis correct density angiogenesis correct
density angiogenesis type of
histogenetic cell classic fibroblasts syncytial
fibroblasts syncytial fibroblasts (dermatoblasts) collagen
architecture dense, non-compliant percolated,
distensible percolated, distensible scar contraction prominent absent absent star chemical collagen glycosaminoglycans glycosaminoglycans (structural) (process regulator) (process and structure) Concerning angiogenesis: Angiocytes
are stimulated by vegf and grow toward the stimulus. In wound healing, the
stimulus is the upper layer macrophages.
They are extrinsic to the tissue in two ways: they are on the surface outside of the forming
new tissue, and they will not be part of the new tissue. Macrophage-angiocyte interaction is open
loop since angiogenesis fails to suppress the macrophages so vessel growth continues
unconstrained. In both Integra
and embryonic histogenesis, the dynamics are different. Angiogenic stimulus is by cells intrinsic
to and distributed internally within the developing tissue. Interaction of angiocytes and stimulatory
syncytial fibroblasts is closed loop because revascularization feeds back to suppress
further angiogenic stimulus. The
result is a tightly controlled vascular density that precisely satisfies
metabolic needs of the tissue. To reiterate, embryonic histogenesis is not directly illustrated
here, but attention is drawn to published sources that have studied this
subject. Of special interest is the research
paper “Ultrastructural Aspects of Human Skin During the Embryonic,
Fetal, Premature, Neonatal, and Adult Periods of Life”, Holbrook KA and Smith
LT, Birth Defects, v.17-2: pp.9 -38, 1981. In this paper, the histology of human
dermal embryogenesis is described in detail, including this quote: “The dermis of the 1-2 month old embryo
is a watery cellular network of mesenchyme that is joined, through long
slender pseudopodia processes and specialized intercellular junctions, into a
syncytium. The undifferentiated
mesenchymal cells at first secrete a matrix that is primarily ground
substance . . . argyrophilic fibrils in the 20-30 nm range are visible at the
light microscope level. . . In the 3rd month, cells separate as the dermis
becomes richer in fibrous components.
Continued deposition of fibers and separation of cells have been
referred to as a ripening of the connective tissue. . . in the fetal dermis
of 14-21 weeks [4-5 months] fibroblasts have assumed a typical spindle shape
and no longer retain cell-to-cell contacts.” This description parallels exactly what is
seen in the early stages of Integra histogenesis. The “. . . watery cellular network of
mesenchyme . . .” refers to the embryonic dermatoblasts which, at least by
light microscopy, share all of the features of the syncytial fibroblasts seen
during “first set” histogenesis in Integra.
While the quoted paper establishes the individuality of those cells by
using electron microscopy, light microscopy gives the appearance of a
syncytium, thus the source of the term “syncytial” fibroblast. Not only are the embryonic dermatoblast and
the syncytial fibroblast the same cell, but never during normal inflammatory
wound healing is such a cell seen. |
||||
|
|
|
|||
|
The embryonic nature of Integra histogenesis as revealed by
histologic features and system dynamics might not be strictly confined to the
matrix itself. Activity of an embryoid
environment might be “broadcast” into the surrounding wound or host as seen
in images such as these. Left, a fibrous musculoskeletal fascia (sural fascia of the leg) subjacent
to regenerating Integra. A normal
mature fibroblast trapped in its collagen matrix seems to have reverted back
to a large regenerative blast form, as witnessed by a mitosis in metaphase,
something not observed in normal wound healing. Right (broad and closeup views),
skeletal muscle fibers subjacent to regenerating Integra (from leg or
thigh). Note the “strings of beads”,
columns of aligned cell nuclei without any apparent intercellular junctions
or cytoplasmic separations. These are
myotubes, true multinucleate syncytial cells that form by the fusion of
primitive myoblasts. Myotubes are a
distinctive feature of embryonic myogenesis, the method whereby long mature
myocytes are created from multiple ordinary small progenitor cells. As central as they are to embryonic muscle development,
they are not seen in normal wound healing or common muscle pathologies and
repair. |
||||
|
|
|
|||
|
Integra histogenesis is characterized by some very distinctive
cells and behaviors: pioneer cells, transitional cells, syncytial fibroblasts
and clusters, and frequently observed mitoses. All of these have morphologies and observed
processes that are very suggestive of embryonic tissue growth and
development. Left, the distinctive cell types of Integra histogenesis are all
seen here, small lymphoid pioneer cells, activated transitional cells that
have bound onto the matrix, large reticulated syncytial cells, and perhaps a
cluster or two. Right, the next
phase in the development of these special cells, the formation of larger
clusters with up to perhaps a dozen cells, active mitoses which account for
the enlarging clusters (not migration and adhesion of wandering cells from
afar, 2 mitoses visible in this one small view), and the incipient production
of collagen and connective proteins, seen as a pale pink blush around or near
the cells. Key to understand is that in normal inflammatory wound healing,
these cells and structures are never seen.
Because wound healing is itself a proliferative process, it is easy enough
to find incidental mitoses of vascular and perivascular cells, but they
typically are seen at the source of the cells, not the destination. Discounting mitoses, the other elements
seen here are characteristic of embryogenesis and Integra, but they are
absent in normal wounds. It could be
argued that pioneer stem cells could be in a normal wound, just overlooked on
standard H&E staining, because they are so similar in appearance to
lymphocytes or the individual nuclear lobes of polymorphonuclear leukocytes. It could be argued that the spindly
appearance of transitional cells could be overlooked in a normal wound
because of the “noise” of so many spindle shaped migratory angiocytes. Fair enough, but the syncytial fibroblast,
the dermatoblast, and its clusters cannot be confused with anything that
belongs in a normal wound. It is
embryonic, and it is not present in a normal wound, ever. |
||||
|
|
|
|||
|
In Integra, the histogenic domain, originated by a single
pioneer cell, is also unlike anything seen ever in a normal wound or inflammatory
wound healing. This structure, which
clusters in a pore of the Integra sponge, has a defined histogenesis and
anatomy that has no comparison in normal wound healing. This is the basic unit of new tissue. It is replicated in each pore of the
sponge. It is unlike scar which acts
as though it was poured or molded into the entire wound space. By analogy, think of a sack that is filled
with glass marbles or metal ball bearings.
If when filled as thoroughly as possible, the sack should retain some
gross malleability or deformability.
In comparison, pour molten glass or metal into that form then let it
solidify. That is the distinction
between scar and Integra. Left, a middle form of the histogenic domain, half way between initial
pioneer cell and eventual mature connective mesh. The original cluster has been vascularized,
allowing second set histogenesis which is just beginning. Syncytial fibroblasts are starting to lose
their original identity, and cell density is increasing a s new more mature
fibroblasts are active and producing young fibrillar collagen. Right, that process has now advanced
to the point of mature final collagen and connective structure. This broader view shows many domains, each
of which regenerated individually, the distributed model of histogenesis,
comparable to embryogenesis and distinct from scar. |
||||
|
|
|
|||
|
In normal subjects, injury begets inflammation begets
inflammatory wound repair and its resulting scar. In contrast is histogenesis within regenerative
matrices, with Integra collagen-gag matrix being the focus of this
presentation. In Integra, the normal
inflammatory response to injury is suppressed. Without inflammation, there is no
inflammatory repair and no scar.
Instead, the material heals by the gradual assembly of a nearly normal
tissue. It is a process which
dynamically and histologically is comparable to normal embryonic
histogenesis. Normal wound healing and
matrix induced embryonic histogenesis do share some features. Their primitive elements are the same –
angiocytes and the vessels they make, and fibroblasts and the connective mesh
they make. However, the commonality
ends there. These two processes fundamentally
alter the appearance, ordering, and assembly of the primitive elements into
two entirely different materials – scar (inflammatory wound healing) versus
neodermis (Integra). The properties
and constituents of the Integra matrix are directly responsible for these
differences, the key factor being the aminoglycans in the material. The physical and biological characteristics
of regenerated Integra are clinically superior to scar and customary skin
grafts, approaching the qualities and mechanics of normal skin. This is not unexpected given the
similarities of Integra and embryonic histogenesis, and their distinction
from inflammatory repair. Matrix regeneration is a new paradigm of surgery. Unlike simple repairs, grafts, and flaps, it
is a fourth paradigm of the surgery of repair, a mode
independent of normal wound healing.
Except for the few introductory cases at the beginning of this
presentation for the sake of orientation, this presentation has not discussed
clinical indications or cases or the technical use of the material. It has focused on the anatomical and physiological
process of Integra regeneration, to explain why it has its special biological
and therapeutic properties. Those
properties, which abrogate or overcome problems inherent in normal wound
healing, mean that Integra has many indications for surgical wound closure
and reconstruction. It is especially effective
for conditions of persistent inflammation where skin grafts and repairs would
lyse or fail, conditions where normal healing would cause problematic
fibrosis and contractures, and conditions of coverage for tissue voids such
as open joints or hardware because of its ability to conduct histogenesis
tangentially through the matrix. For
certain types of problems, use of a regenerative matrix such as Integra should
not be considered as a novel alternative but rather as the primary modality,
the indicated option for cases where inflammation, scar and contractures, and
problematic coverage put normal wound-healing-dependent surgery at risk. |
||||
|
|
|
|||
|
The use of regenerative matrices in surgery is a form of tissue
engineering that is conducted in situ on the host, on the target wound or
reconstruction. The process of
histogenic new tissue formation has been elucidated histologically. It is an integrated sequence of interactions of
certain cells and the substructures they make. Since they are making a connective tissue,
only four elements contribute to the final product, angiocytes, fibroblasts,
blood vessels, connective mesh. These
same four elements make all of the connective structures, from scar to fascia
to dermis, the differences between these tissues being in when these four
elements and their subassemblies appear and how they arrange themselves. The physics of these processes, the timewise
and spatial dynamical interactions of these elements, govern the
characteristics of the final structure.
For normal wound healing, it is scar.
For Integra collagen-gag matrix, it is a dermal analogue with entirely
different mechanical properties. The
Integra process does this by suppressing normal inflammation and wound
healing and inducing an embryonic form of regeneration. This confers benefits that solve clinical problems
when classical paradigms of surgery will not work. Right, a view of Naples (Napoli), Italy from the
lighthouse on the molo (pier), original art by Joseph Mallord William Turner (1775–1851),
engraved by G. Cooke, 1820. At the
left edge is the Castel Nuovo (first built 1279), and in the back on the hill
is the Castel Sant’Elmo (structure built incrementally 1275-1547). It reminds that classical wound healing is
a foundational function of multicellular life, robust, protective, the basis
of our entire surgical civilization. Left, a
view of a covered wagon train traveling under Pilot Knob, 1872. It is located in southern California near
the Colorado River bordering Arizona.
Circa 1850-1880, before the railroads entered and crossed the Arizona
Territory, access to Arizona was from the west via by then populous California,
from the east by dangerous stage coach or wagon trains, or by ship across
Panama or around Cape Horn to the Gulf of California to the mouth of the
Colorado. Pilot Knob, a volcanic form with
its distinctive shape, was an important landmark in those years. It was a guide for the overland stages and
trains of the Southern Emigrant Trail during
the California gold rush, and for the riverboat traffic
that was so vital to Arizona in its early years. It is a reminder that something can be
built from bare landscape, whether on a continental scale or just within a
small piece of regenerative biomatrix. |
||||
|
|
|
|||
|
END - FINE |
||||
|
|
|
|||
|
|
||||