|
Table 2b.
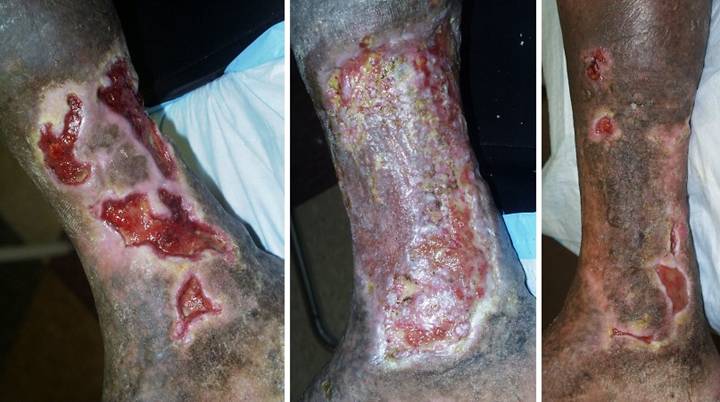
Ulcer Anatomy, site and complications
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
2b. This table profiles the anatomy of the ulcers, their site
and complications. Data are again stratified by primary diagnosis. Unlike the previous tables which analyzed
the 111 patients, this table focuses on the 166 ulcers that were managed with
Integra in those 111 patients. These 166 ulcers are partitioned by major
anatomical region. “Anatomical
complications” refer to exposure of internal structures, including bones,
joints, tendons, and internal organs, their incidence listed as number of
patients who had these conditions (rather than the number of individual anatomical
structures exposed). The incidence of
exposed structures was higher than listed, but analysis was limited to
verifiable information in the available medical records. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
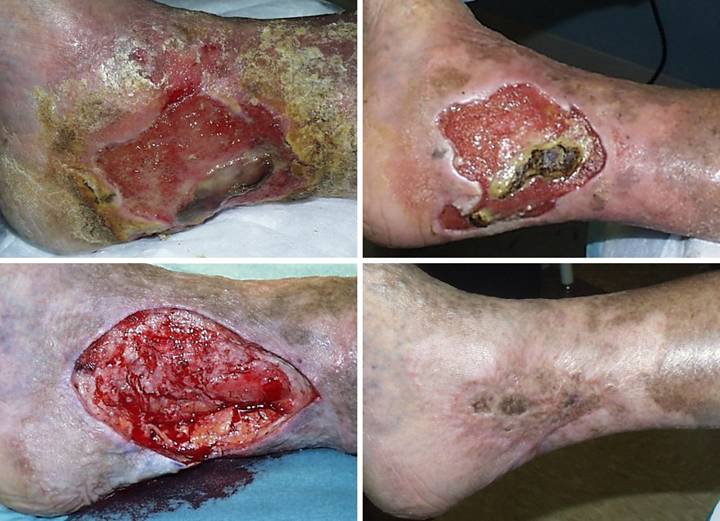
Table 2c.
Ulcer Anatomy, detailed
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
2c. This table shows more detailed information about the
location of the ulcers. All of the 166
documented ulcers, “instances”, are partitioned by anatomical location. Note that the sum of “number of patients”
having ulcers at a given location is undefined, exceeding the 111 study
patients because some had ulcers at multiple locations. |
|
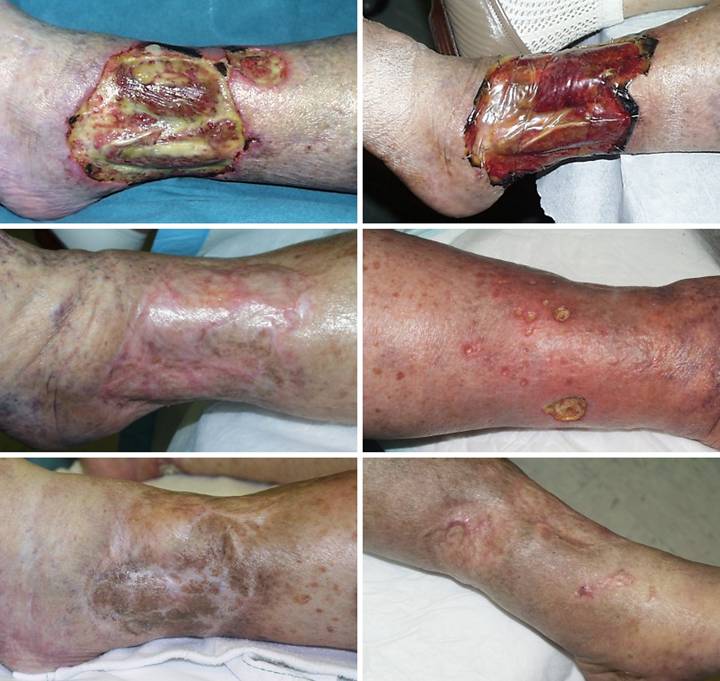
Table 2d.
Ulcer Complications, detailed
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
2d. This is a more detailed profile of the exposed internal
structures (in 78 patients) that were covered by Integra. The posted data are again limited to only
those exposed structures explicitly documented in operative and clinic
records. Exposed bone is partitioned
by whether only cortical bone was covered, or if debridement or ostectomy had
exposed cancellous bone. “Open joint”
refers explicitly to joints in which there was an arthrotomy with loss of
joint capsule and synovium and exposure of the joint space. Major / minor tendons are classed based on
physical size or functional significance.
Grouped tendons such as the finger and toe extensors are counted only
once for each instance, even though multiple individual tendons might have
been exposed. Many of the treated
wounds had additional undocumented exposure of minor tendons. Integra also covered many joint and
retinacular ligaments, structures which also might ordinarily be closed by
flaps, but the incidence of these situations was undocumented in the
available records. Many pieces of
Integra were applied directly to large areas of muscle, but because of the
dependably good results, there was no special attention to this condition,
neither clinical nor analytical, and instances and results are not
tabulated. Coverage of viscera and
hardware are presented in the case studies.
The 173 instances of exposure occurred in 111 individual ulcers ( 67%
of all ulcers) in 78 patients (71% of all patients), reflecting the severity
or complexity of most of these problems. |
|
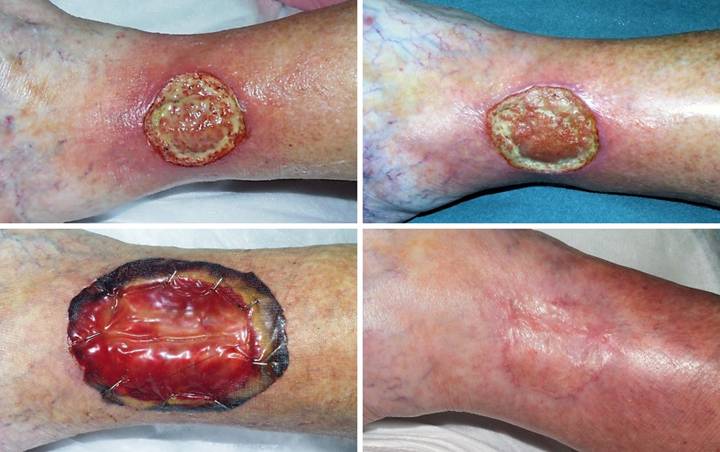
Table 3a. Outcomes, by outcome category and
complications
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
3a. Patient survival and available data permit outcome
analysis in 103 of the 111
patients. Outcomes are divided into
three general groups. Group 1 are
those patients in whom Integra resolved the chronic ulcer, subdivided into 4
scenarios. Group1a is the nominal
uncomplicated Integra reconstruction (excise the wound, place Integra, place
skin grafts when regenerated, healed).
As with any skin graft, a few weeks of topical care may be required
for complete healing, but in some patients, group 1b, persistent open areas
after the epidermal overgrafts required more than 8 weeks of care, usually
with additional topical modalities such as PDGF (platelet derived growth
factor) to coax complete reepithelialization of the regenerated Integra. In group 1c, incomplete skin graft take and
reepithelialization prompted a second skin graft over the original Integra in
order to complete the reconstruction.
In group 1d, Integra reconstruction was successful but incomplete, and
residual open areas or areas of lost Integra were successfully closed with a
second application of Integra (and subsequent skin grafts). Group
2 are those patients in whom Integra incompletely healed the wound but
nevertheless contributed to a successful wound and patient outcome. Most of these patients had successful
closure of part or most of the wound with Integra, but a more conventional wound
closure (repair, skin grafts, flaps) was needed to finish select areas or
persistent open structures (group 2a).
Integra’s ability to suppress inflammation and ameliorate adverse or
pathological conditions in the wound was important in three patients, leading
to a stable asymptomatic non-pathological wound that could be managed or
closed by other means (2b). In two
patients (2c), skin grafts over Integra were unstable, leading to persistent
open regenerated Integra, but the wounds and patients were still
substantially improved because wound pathology and symptoms were controlled
and manageable by chronic topical care. Group
3 are the failures, including loss or failure of the Integra with a
persistent wound (group 3a), persistence or progression of ulcerative wound
pathology (group 3b), and failure of the reconstruction leading to amputation
(group 3c, 2 above knee, 3 below knee).
One infection occurred (inflammation, pain, suppuration, graft lysis,
fever), but it is not formally tabulated because it occurred in one of the 8
patients with an unassessable outcome.
Integra performed properly in some of the amputation patients, but
they are tabulated as failures anyway because the Integra reconstruction did
not contribute to an ultimate good outcome. |
|
Table 3b. Outcomes, by diagnosis
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
3b. In this table, the 103 outcomes in three general outcome
categories are stratified by underlying diagnosis. In the incompletely healed Group 2, Integra
was of greater or lesser success from one patient to another. The relative success is ranked by the
approximate area, in thirds, of the original wound which was successfully
closed (note that Group 2 subdivisions x, y, z are distinct from the outcome
types a, b, c, d in table 3a). The
predominant cause of failure was atherosclerotic arterial insufficiency. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3c. Outcomes, by site
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
3c. In this table, the 103 patient outcomes in three general
outcome categories are stratified by anatomical site. Recall from table 2c that many patients had
multiple ulcers. The entry “multiple”
refers to patients that had ulcers at more than just one of the sites listed
in the other rows (all of these combinations were on the lower extremity, e.g.
leg and ankle, or leg and foot). |
|
Table 3d. Outcomes, closure of internal structures
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
3d. There were 173 exposed structures among all 111 study
patients, of which 166 occurred in the 103 patients included in the outcomes
analysis. This table lists the results
of Integra applied over internal structures for those 166 instances. The number-of-patients column reflects that
some patients had multiple exposed structures. Of the 166 instances of exposed structures
with known outcomes, 90% were successfully closed by Integra. Ten structures (6%) were eventually closed
with secondary surgery, but Integra functioned as a competent artificial
skin, keeping those structures safe, thereby permitting late closure and
salvage. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table 4.
Length of treatment
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table
4. This table shows the duration of treatment. “Integra-to-skin grafts” is the regeneration time in weeks, the mean
times between placing Integra and placing the first set of skin grafts,
precise information available for 99 patients. The intervals were counted in days, then
converted to weeks for display. Since
this parameter was largely controlled by the surgeon and the basic physiology
of Integra, the values and distributions are relatively uniform. The exception is the malignancy category,
where, as would be expected, the
radiation patients caused a high end bimodal spread. “Integra-to-healed”
is the interval, in months, between placing Integra and when the wound was
fully epithelialized. This information
could be ascertained accurately for 70 patients. Patients in the Group 2 partial success
category are also included, their interval measured by when secondary grafts
or flaps were healed. Data is
presented for patients, not ulcers.
For patients with multiple wounds, the times for individual ulcers
were averaged, and that was used as the value for each patient. Patient values were then averaged within
each diagnostic category. In four
groups, there was a clear outlier which was excluded from the averages (the
parenthetical values under “range” are the four outliers). The total value is the direct average on
all 70 patients. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Table 5.
Inpatient versus Outpatient
|
||||||||||||||||||||||||||||||||||||
|
Table
5. This table estimates the economics of using Integra by
counting the number of patients who had inpatient hospitalization versus
those whose care was entirely outpatient.
The first data column is the number of study patients each year
(because the study covers only 6 months of 1996 and 2002, the numbers in
parentheses are pro rata annualizations so that the overall trend can be more
easily visualized. The second column
is the number of inpatients who had an inpatient admission at any time
related to their Integra reconstruction, either with the primary excision and
Integra or the skin grafts. “Inpatient
admission” is defined as a formal hospitalization of greater than 24 hours
duration subject to the legal and administrative criteria of inpatient
reimbursement, and does not include overnight stays of less than 24
hours. The last column is the
percentage inpatients, the ratio of the first two columns. The group inpatient rate was 36% over 6
years, but there was an important trend.
The inpatient rate was a linear decline to zero (linear regression on
the last column has r2 = 0.9698).
The problems and severity of the patients were not obviously changed,
and this decrease reflects multiple factors as described in the discussion. |
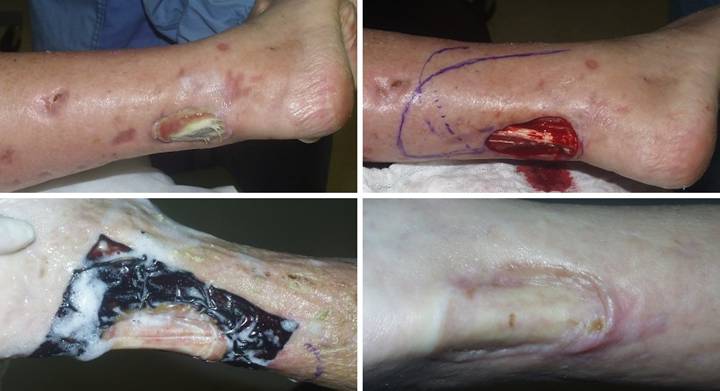
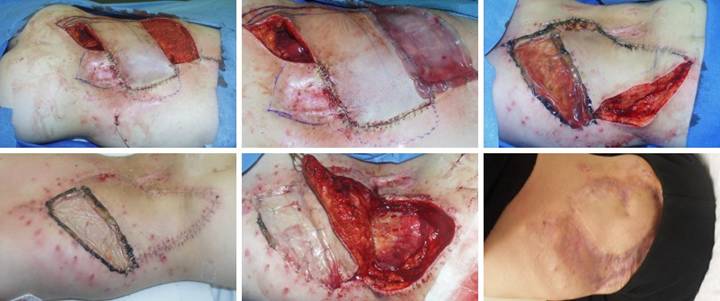
CASE STUDIES
|
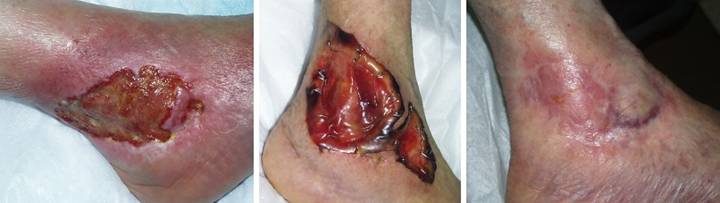
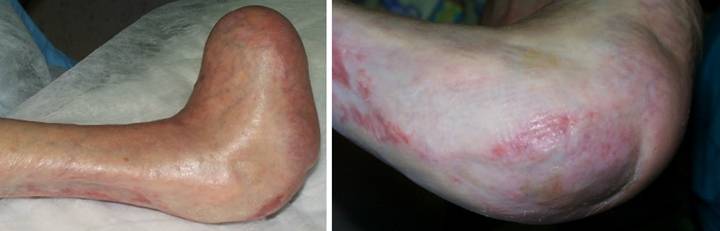
Case Study 1, Outcome type 1a, nominal reconstruction,
healed.
A 65 year old woman with Wegener’s granulomatosis and severe related
pulmonary disease developed an achilles ulcer. (Figure 1a) A period of wound care
and stabilization of disease induced some “granulation tissue”, a
proliferative wound module, indicative of wound healing competence. However, because of tendon shearing, this
wound cannot heal easily, even in a healthy person, and with active disease,
risks to wound and patient are high. (Figure
2b) In surgery, the wound was
excised, and the tendon was decorticated until only healthy fibers
remained. The blue lines are the
design for a reverse sural nerve flap and donor site skin grafts, considered
then rejected in favor of what was safest.
(Figure 1c) The material is shown here regenerated and ready
for skin grafts. (Figure 1d)
Two months later, the tendon is healed and stable. Figure 1 a (top
left), b (top right), c (bottom left), d (bottom right)
|
|
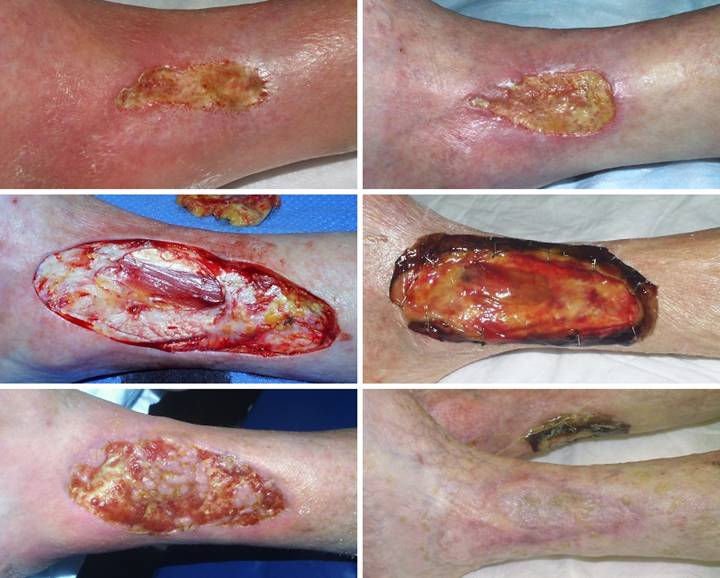
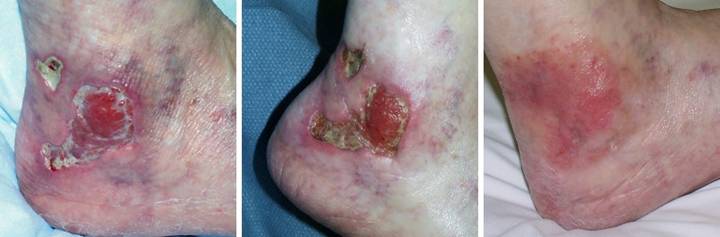
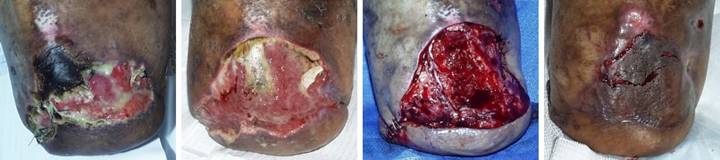
Case
Study 2,
Outcome type 1b, healed after ancillary care. A 69 year old
woman with severe rheumatoid arthritis developed an ankle ulcer following
prosthetic knee arthroplasty. (Figure
2a) There was periwound inflammation and lytic necrosis of the margins,
typical of rheumatoid and hypercoagulable ulceration. Laboratory diagnosis revealed Factor V
Leiden heterozygous, low proteins C and S, and high homocysteine. Arterial ankle-brachial index was 0.93, and
periwound transcutaneous oxygen pressures were 4 – 50, increasing to 200 – 330
breathing 100% O2. These
studies confirm a hypercoagulable disorder without arterial macrovascular
disease. (Figure 2b) It failed
to improve significantly after 6 weeks of basic wound care, anti-inflammatory
therapy, and warfarin. (Figure 2c)
In surgery, excision of the wound and
surrounding calcinosis cutis exposed muscles, ligaments, and tendons. Integra was opted for closure for reasons
of disease stability and essential coverage.
(Figure 2d) After placement of Integra, overt inflammation
completely ceased, and the material regenerated. (Figure 2e) Two months after skin
grafts, epithelialization was retarded, with only small islands of epidermis
scattered over the regenerated Integra.
Platelet derived growth factor was initiated, after which the skin
closed. Note that even though not yet
completely healed, that the regenerated Integra is a healthy tissue, without
necrosis-lysis-ulceration nor periwound inflammation. (Figure 2f) One year later, the
patient had a severe rheumatoid flare-up, with multifocal new foot and leg
ulcers. The original left leg
reconstruction remained healthy and uninvolved. Integra was used on the new ulcers, seen in
the back over the right achilles. Figure 2 a (top
left), b (top right), c (middle left), d (middle right), e (bottom left), f
(bottom right)
|
|
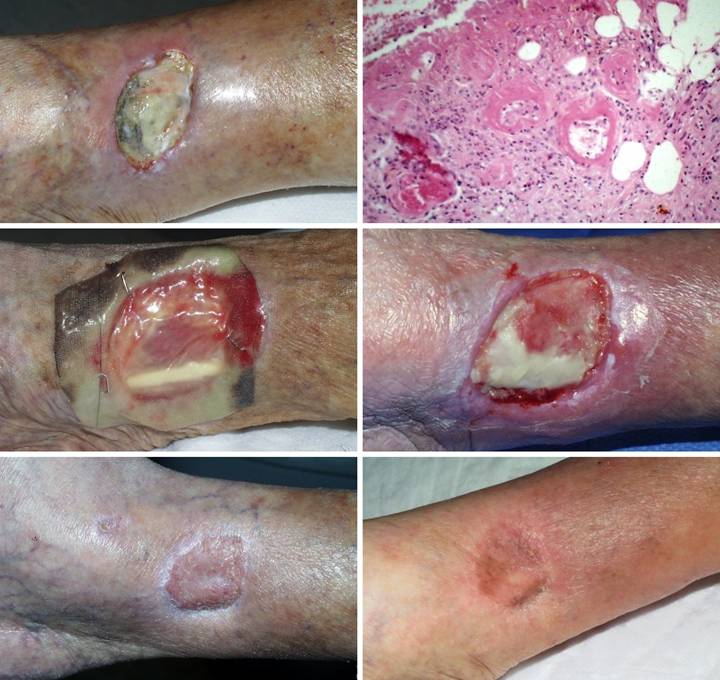
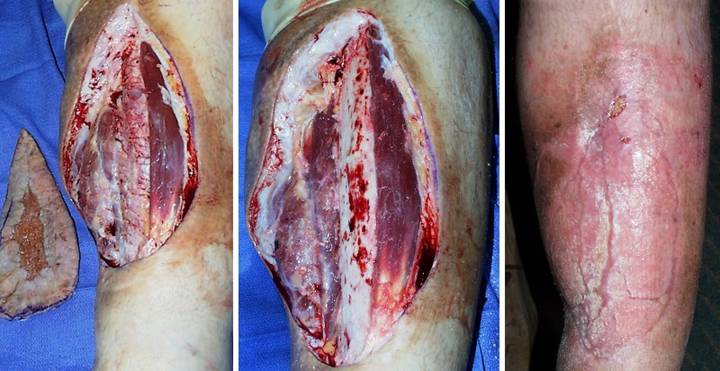
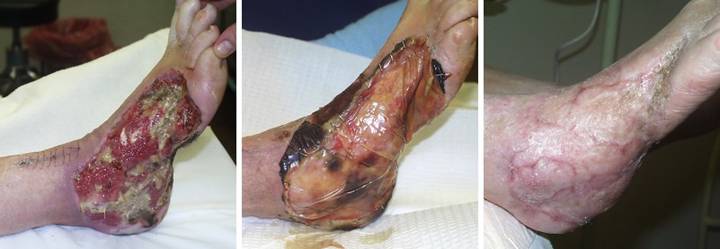
Case
Study 3,
Outcome type 1d, healed after second Integra. A 69 year old
woman presented with a small ulcer over a varicose vein, right medial
leg. Arterial circulation was
normal. Failing to heal with basic
care, it was excised and sutured. (Figure
3a) The sutured wound became
necrotic. Active ulceration at the
margins progressed in spite of good topical care. (Figure 3b) Workup for pathology revealed protein C deficiency and cryoglobulins,
corroborated by histology which showed stasis, thrombosis, vascular necrosis,
and only sparse inflammation, findings typical of microthrombotic
ulcers. Warfarin anticoagulation was
started. (Figure 3c) When the ulcer still did not improve, the
wound was excised. Skin grafts and
local flaps were contraindicated because they would have the same
complications that caused the ulcer in the first place. Integra closed the wound and controlled
pathology, with no further necrosis. (Figure
3d) By the time the Integra was ready to lose its silicone, it had not
yet regenerated over the flexor digitorum longus tendon. Because the matrix can conduct tissue, the
tendon will close, but it needed more time.
This is not a failure, but merely the time to apply a second serial
piece of Integra. (Figure 3e)
Three months after skin grafts, all was healed. The contours of the tibialis posterior
muscle and the flexor digitorum tendon are easily seen. (Figure 3f) Two years after that,
the reconstruction remains healthy. Figure 3 a (top
left), b (top right), c (middle left), d (middle right), e (bottom left), f
(bottom right)
|
|
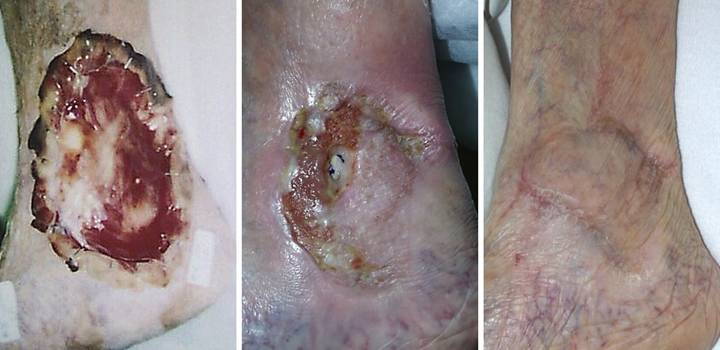
Case Study 4, Outcome type 2a, partial success, healed
after secondary flap. An 86 year old woman presented with chronic
right medial ankle ulceration of several years duration, probably just
venous, but complicated by exposure of major tendons and the ankle
joint. (Figure 4a) After
excision, Integra was used to close the wound and its various exposed
structures. (Figure 4b) Integra
healed the wound except for a small perforation into the tibialis posterior
tendon sheath. Unepithelialized
surfaces seen here would have healed within a few weeks, but the shearing
tendon (its excursion marked by blue dots) requires explicit closure. (Figure 4c) The reconstruction was
completed by using a small flap from the dorsum of the foot to cover the
tendon. Note the normal quality of the
Integra skin, soft, compliant, wrinkling and folding with ankle motion. Figure 4, a (left),
b (middle), c (right)
|
|
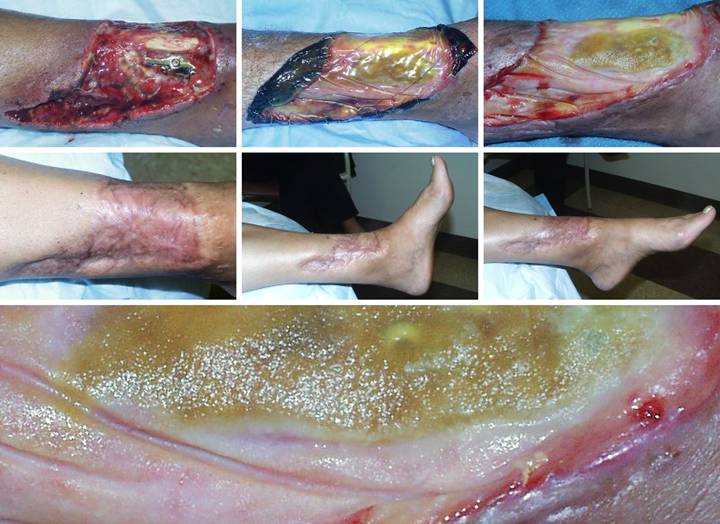
Case
Study 5,
Outcome type 2c, persistent open Integra. A 75 year old man
had extensive chronic venous disease and ulceration of both legs,
unresponsive to all treatments over many years. (Figure 5a) The right medial leg has the usual stigmata
of severe long standing venous hypertension and chronic stasis dermatitis,
with pronounced dermatosclerosis and ulceration. (Figure 5b) All pathological skin, fascias, and wounds
were excised, veins were stripped, and skin was reconstructed with Integra. Seen here shortly after skin grafting, the
Integra is completely regenerated.
Small areas of incomplete skin graft are healthy, with prompt complete
reepithelialization expected. (Figure
5c) Due to special circumstances,
the patient had to return quickly to his usual work, and he was unable to
continue complying with all prescribed care.
Although complete closure was anticipated (especially since this was
just venous disease), stasis effects recurred, and some areas never
healed. Even the Integra reconstructed
skin developed venous pigmentation, shown here one year later. His care has capitulated to a program of
long term maintenance for the remaining open areas. While this is not an
ideal result, patients are much more accepting of big improvements than their
physicians are of anything not perfect.
Integra was successful, because compared to what he had, he is much
improved, with chronic inflammation, drainage, and pain eliminated, and daily
function preserved. Figure 5, a (left),
b (middle), c (right)
|
|
Case
Study 6,
Diagnosis, venous. A 77 year old man with chronic venous
ulceration presented after yet another failed skin graft. Most venous ulcers will heal within the
traditional scope of management: good topical care and compression, followed
by venous interruption, and then excision and skin grafts if needed. However, when disease is persistent, other
care has failed, and there are essential coverage needs, then excision and
skin reconstruction is required. (Figure
6a) Upon presentation, the right
medial ankle ulcer was surrounded by extensive eczematous dermatitis and
edema. Necrotic recent skin grafts
were still present. (Figure 6b) After two weeks of basic hygiene, topical
steroids, and compression, acute conditions were controlled. Because of the given history, healing was
not expected with topical care and compression alone. (Figure 6c) Ulcer excision exposed the posterior tibial
neurovascular bundle, posterior compartment muscles, and the achilles fat
space. Integra was opted for closure
for all of the reasons mentioned: chronicity, a history of failed care, and
the presence of exposed structures and adverse biomechanics which would
prevent successful skin grafting. (Figure
6d) In conjunction with continued
elastic compression, the reconstruction has remained healed and healthy, seen
here at one year. Figure 6 a (top
left), b (top right), c (bottom left), d (bottom right)
|
|
Case
Study 7,
Diagnosis, immunopathic. A 71 year old
woman had an ulcer of the lateral left leg and ankle. (Figure 7a) She was treated for 4 years for “venous
ulceration”, but multifocal lytic ulceration, exposure but sparing of muscles
and tendons, distribution along tendon sheaths, absence of venous pigment and
edema, and various musculoskeletal symptoms are all paradigm features of
rheumatoid and similar immunopathic wounds.
(Figure 7b) After making
and confirming the diagnosis of rheumatoid arthritis, anti-inflammatory and
antimetabolic treatments were started.
The wound was excised and closed with Integra, including coverage of
bare fibular cortex and multiple muscles and tendons. (Figure 7c) The involved area healed quickly and has
remained healed ever since, seen here at 6 months. (Figure 7d) Two years later, the patient became very
ill with disease flare-up. Rheumatoid
panniculitis and multifocal ulceration occurred on both lower extremities,
seen here on the medial side of the left leg.
Topical wound care, compression and edema control, intralesional
steroids, and increased systemic therapy brought the acute problems under
control, but left multiple open wounds.
(Figure 7e) This image
is simultaneous to figure 7d. Whatever
else has happened nearby, the original lateral leg reconstruction remained
healthy throughout the acute flare-up.
(Figure 7f) The new
ulcers on the medial side of the leg did not heal after a suitable period of
topical care and observation. Integra
was then used, and all healed, seen here 9 months later. Figure 7 a (top
left), b (top right), c (middle left), d (middle right), e (bottom left), f
(bottom right)
|
|
Case
Study 8,
Diagnosis, hypercoagulable. A 61 year old woman had left leg
ulceration of many years duration and a history of multiple venous
thrombosis, pulmonary embolism, and warfarin resistance. These are hallmark features of a
hypercoagulable disorder, but long standing warfarin therapy precluded making
the exact pre-thrombotic diagnosis. (Figure
8a) In spite of the history, the
usual stigmata of venous disease, pigment, edema, and dermatosclerosis, were
not very severe, and there was never any improvement by compression and
topical care alone. This was a
hypercoagulable rather than a venous ulcer, further confirmed histologically
by microthrombi. Granulation tissue at
the base of the wound indicated intrinsic wound healing competence, but there
was chronic active dermatitis, panniculitis, and necrosis in the margins and
periwound soft tissues. (Figure 8b) After a few weeks of hygiene, good topical
care, compression, and increased warfarin, the wound and periwound were
improved, but nevertheless, inflammation and active necrosis-ulceration
persisted at the margins. (Figure
8c) In surgery, the ulcer was
excised, including a tangential fibular ostectomy for hyperplastic
osteophytes (common under chronic inflammatory ulcers, due to the effects of
transforming or pro-proliferative growth factors which are perpetually in the
wound). The wound was closed with
Integra. Seen here at 6 days,
periwound inflammation, erythema, and edema have completely subsided. (Figure 8d) The ulcer remained healed during a 4 year
follow-up period, seen here 9 months after skin grafts. Figure 8 a (top
left), b (top right), c (bottom left), d (bottom right)
|
|
Case
Study 9,
Diagnosis, mechanical. A 79 year old woman presented with an ankle
ulcer of several years duration. (Figure
9a) The right lateral malleolar
area was inflamed with eczematous dermatitis.
The wound base had granulation tissue indicative of sufficient
arterial circulation and intrinsic wound healing competence. However, there was persistent active
ulceration and failure of epithelial ingrowth. Workup for all of the usual
culprits failed to make any diagnosis other than chronic pseudarthrosis at an
old malleolar non-union directly under the ulcer. The significance of tissue mechanics and
their influence on mesenchymal differentiation, repair, and pathology should
never be overlooked. The cardinal
signs of pseudarthrosis are inflammation and pain, and in a susceptible
elderly person, it can create enough local pathology to maintain an active
ulcer. (Figure 9b) The ulcer did not close with improved
topical care and splints, so surgery was done. It was excised, including bone fragments
and the arthrosis, and Integra was used to close the wound and the structures
underneath. Seen here 11 days later,
periwound inflammation is gone. (Figure
c) The wound healed and has
remained so, seen here a year later. Figure 9, a (left),
b (middle), c (right)
|
|
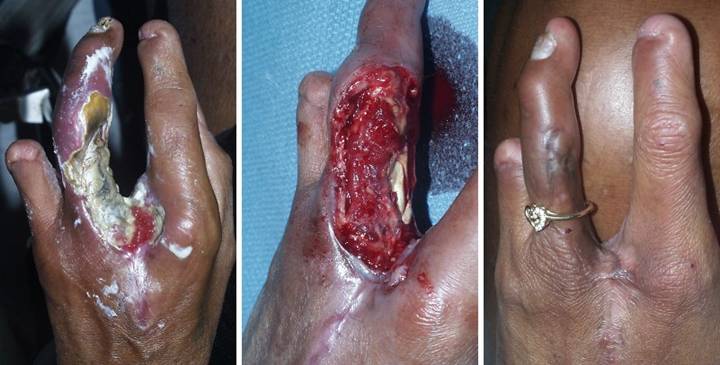
Case
Study 10, Location, upper extremity. A 42 year old
woman with diabetes and upper extremity atherosclerosis developed ischemic
ulceration of the left long finger.
Progressive necrosis and ray amputation resulted in a mid hand wound
and partial necrosis of the ring finger.
(Figure 10a) After a few
weeks of good wound hygiene, silver sulfadiazine, and debridement, the wounds
stabilized, and necrosis and further ulceration were arrested. Arterial pressure and circulation were not
as bad as first thought, evidenced by the completely healed central
hand. Granulation tissue attests to
active wound healing and a potentially salvageable ring finger, as long as
essential coverage issues over the skeletal structures can be fulfilled. The usual flaps from adjacent fingers
cannot be done in this high risk arteriopathic hand. (Figure 10b) After excisional debridement, specific
structures needing coverage were the web space, the proximal interphalangeal
joint, and the flexor tendons and their sheath. (Figure 10c) The hand healed. Integra closed the flexor tendons, and it
reconstructed a fully compliant web space free of scar and contracture. The interphalangeal joint had a persistent
small ulcer which was closed with a small secondary flap from the dorsum of
the joint. Joint motion is limited,
but the patient eschewed therapy and is very happy to have a healed hand
without having lost the ring finger. Figure 10, a
(left), b (middle), c (right)
|
|
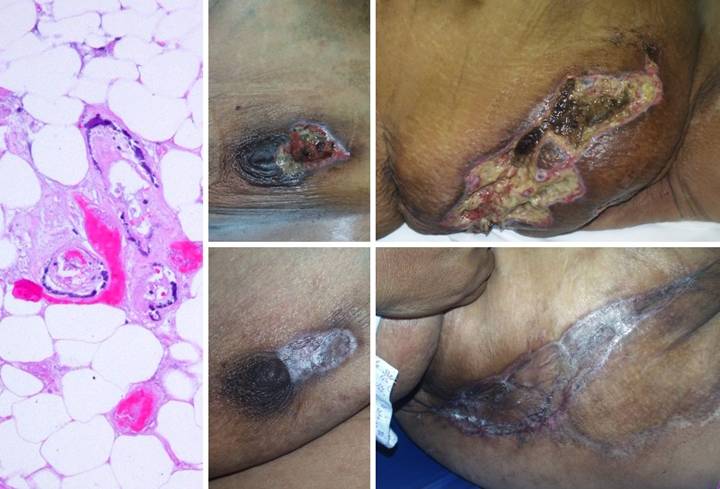
Case
Study 11, Location, trunk. A 51 year old
woman, with dialysis dependent renal disease and tertiary
hyperparathyroidism, developed multifocal necrosis and ulceration of the
trunk. (Figure 11a) The history, pattern of involvement, and
the debilitating ischemic pain were all typical of systemic calcinosis
(calciphylaxis). The diagnosis is
easily made by clinical features, but histology of debrided material confirms
it. Medial arteriosclerosis of all
small order arteries and arterioles is characteristic, often accompanied by
microthrombosis. (Figures 11b,c) The patient had numerous infarcted lesions
on the trunk. The right breast and
right flank (lower abdomen) are shown.
This condition is refractory to usual topical and surgical care. Progressive necrosis is common, and
attempts to debride can cause more necrosis (pathergy). Managed by good topical care alone, many
months may be required for closure.
Skin grafts and customary repairs are likely to fail or cause more
problems. (Figures 11d,e) All necrotic areas were excised and closed
with Integra. Pain and progressive
ulceration immediately ceased. All
areas healed quickly, seen here 3 months after excision and Integra. This is the paradigm Integra
reconstruction, complete success without delays. Integra’s ability to close and control the
wound without donor sites nor risk to the patient is ideally suited to this
diagnosis. Figure 11 a (left),
b (top middle), c (top right), d (bottom middle), e (bottom right)
|
|
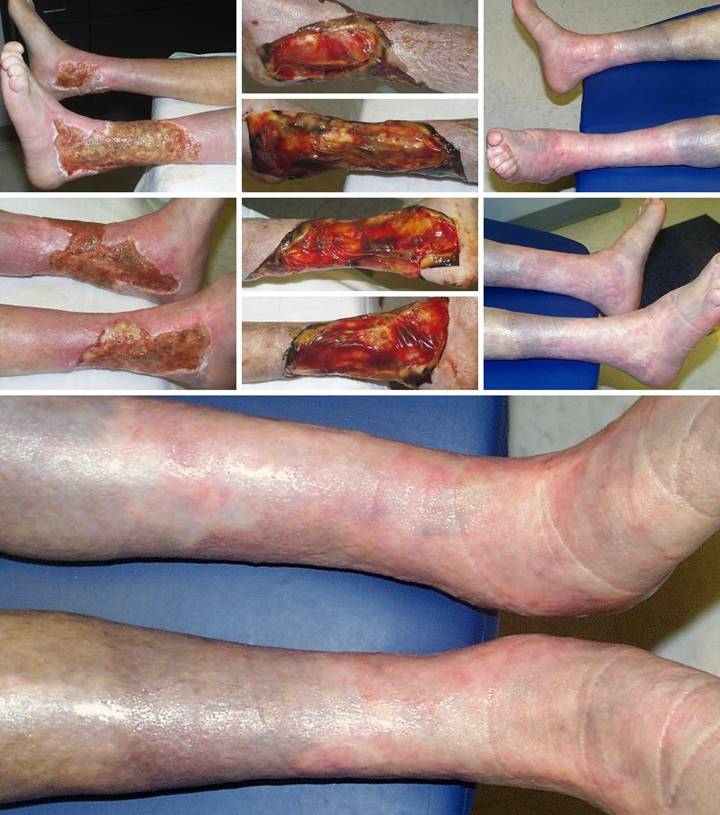
Case
Study 12, Location, leg. A 77 year old
woman had a 40 year history of continuous leg ulceration and progressive
systemic illness. In spite of classic
symptoms of Sjögren’s disease, the diagnosis was missed countless times. When seen in consultation, the diagnosis
was made, anti-inflammatory and antimetabolic treatments were started, and
the patient’s general health status improved substantially. (Figures 12a,b) The leg ulcers are shown just prior to
excision. Proper excision means
thorough fasciectomy, removing all pathological tissues, including the
fibrotic, ulcerated, and inflamed sural fascia. This means that muscles, tendons,
retinacular ligaments and other structures will all be exposed when excision
is complete. Skin grafts will not
cover these structures, and even if they could in principle, here they would be
at risk for recurrent pathological lysis and ulceration. (Figures 12c,d) The legs and ankles are shown one week
after excision and Integra. Note the
wrinkling in the Integra, a common occurrence due to control of inflammation and edema, thereby decreasing
limb volume and surface. All care was
outpatient. (Figures 12e,f) The legs healed and have stayed stable,
shown here two years later. Note the
bandaging imprints, attesting to the patient’s diligent efforts to control
edema and care for her skin.
Consistent rheumatology management has kept the patient healthy, and
lifestyle has been restored. (Figure
12g) A close-up view shows the
quality and texture of the regenerated material and how comparable it is to
normal skin. Figure 12 a, b
(left column), c, d (middle column), e, f (right column), g (bottom)
|
|
Case
Study 13, Location, foot & ankle. A 77 year old man
with long standing rheumatoid arthritis developed a right lateral ankle
ulcer. (Figure 13a) The wound was a typical rheumatoid lesion,
characterized by multifocal lysis of skin and fascias. (Figure 13b) After a month of various care, the wound
did not improve, with inflammation and marginal necrosis still active. With spontaneous improvement not expected,
surgery was required. In a healthy
patient, skin grafts or dependable local flaps would have been best. In this situation, challenged by active
rheumatoid disease, and given the contiguity with mechanically active
structures, Integra was the safest and surest option. (Figure 13c) The Integra reconstruction healed quickly,
seen here after a few months. In this
image, there is some contact dermatitis due to prolonged use of dressing
materials after everything was healed.
The Integra reconstructed skin is inherently healthy, and dermatitis
cleared promptly with topical steroids. Figure 13, a
(left), b (middle), c (right)
|
|
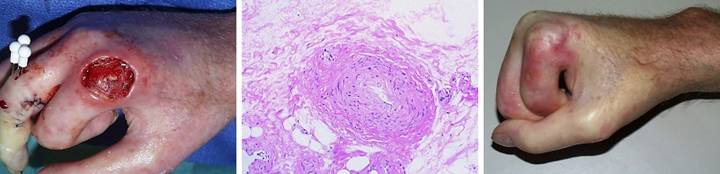
Case
Study 14, Exposed structure, bone. A 33 year old man
had chronic recurrent venous ulceration of the leg following femur fracture
and femoral vein injury at age 14. (Figure
14a) Surgery was opted after
repetitive cycles of disease made it clear that the skin and scars were
unstable and would be perpetually ulcerated in spite of care. All pathological tissues were excised,
exposing inflammatory dysplasia of the tibial cortex. (Figure 14b) The tibia was planed back to
architecturally normal bone. Integra
was used to cover the tibia and reconstruct healthy new skin over the entire
wound. (Figure 14c) At 5 months, the reconstruction is almost
completely healed (it has since healed and has remained stable for 5 years). Figure 14, a
(left), b (middle), c (right)
|
|
Case
Study 15, Exposed structure, joint. A 43 year old man
developed multiple complications of rapidly progressive scleroderma. (Figure 15a) His hands had skin atrophy, sclerosis, and
telangiectasias typical of scleroderma.
There were multiple contractures and skin lesions. The largest ulcer was on the dorsum of the
index finger metacarpophalangeal joint, where loss of the dorsal joint
capsule caused direct wide exposure of the joint space and dorsal bone
surfaces. As one of the few fingers
not contracted and still functional, salvage was important for rudimentary
activities. This view, in surgery,
shows the open metacarpophalangeal joint and degenerated bone at the base of
the phalanx. (Figure 15b) These open structures required suitable
coverage. Sclerotic skin made local
flaps a technical impossibility.
Histology showed stenotic fibrotic arteries typical of immunopathic
angiopathy, and impaired circulation made any surgery risky. Conventional options for closure, including
topical care, repair, flaps, grafts, and amputation, were all too risky,
doomed to fail, technically unfeasible, or too destructive of remaining
function. Only Integra offered a safe,
dependable, sensible solution. (Figure
15c) Integra healed the open bones
and joint and preserved a functioning finger, shown here at 6 months. The material is compliant enough to allow
full flexion. Threats to the finger
are gone, and daily function is possible. Figure 15, a
(left), b (middle), c (right)
|
|
Case
Study 16, Exposed structure, hardware. A 50 year old
woman had a distal tibia fracture treated by plate and screw fixation. Skin dehiscence was managed by rectus
abdominis and latissimus free flaps, both of which died. (Figure 16a) When seen in consultation, the first jobs
were to stop all other surgery, clean up the wound, and work her up for
autoimmune and hematological disorders as suggested by the history,
pathological wound behavior, and necrotic skin margins (an explicit diagnosis
was not established). The tibialis
anterior tendon, the metal plate and screws, and the fracture underneath were
all exposed and needed a solution for coverage and salvage. Options for surgical closure of an open
distal tibia are already limited, and in this case, all choices, local flaps
and free flaps, carried substantial risk of further failure. The patient was averse to any other
elaborate surgery which carried risk.
Rather than take these risks, Integra was opted. (Figure 16b) Integra was not intended to be the means of
final skin closure. It was used to take advantage of its role as a high
quality artificial skin. The goal was
to stay ahead of silicone separation, replacing a new piece every 4 weeks
until the fracture was healed and the plate could be removed, probably after
3 or 4 months. The first piece of
Integra is seen here at the first 4-week exchange. It has regenerated over non-essential soft
tissues, and it is regenerating over the tibialis tendon. The leg is otherwise healthy, free of edema
and necrosis, showing edema-reduction wrinkles in skin and Integra. (Figure 16c) This image shows the matrix after removing
the silicone. The matrix over the
plate is intact, filled with yellow serum.
At the margins of this zone, tissue is starting to grow inward,
tangentially across the matrix. Skin
grafts were placed on peripheral regenerated areas, and new Integra was
placed over the central zone. (Figure
16d) Tangential histogenesis as a
means of complete regeneration was not the original surgical plan, but that
is what happened. There were no
complications nor setbacks of any kind.
The patient became quite skilled with the required care, permitting
her to take an out-of-country holiday vacation for three weeks while the
third piece of Integra was in place.
After the third Integra (and skin grafts), the leg was healed, seen
here 11 months after starting the reconstruction. It has remained healed for two years, the
hardware uncomplicated and still in place.
(Figures 16e,f) The
patient is fully ambulatory and active, with no restrictions of any
kind. The fracture is healed, and the
plate and screws remain in place.
Ankle dorsiflexion is off by 20 degrees, but this is a consequence of
trauma, not the reconstruction, and it is better than having the whole foot
off. Plantar flexion is normal. (Figure 16g) This image is a close-up view of the matrix
at the time of the first exchange.
Opacified new tissue is diffusing inward across the matrix from the
living margins. This centripetal
growth will continue until the entire matrix has generated tissue. Figure 16 a (top
left), b (top middle), c (top right), d (middle left), e (center), f (middle
right), g (bottom)
|
|
Case Study 17, Adjunct use, flap delay and donor site. A 14 year old boy
had a back ulcer of several years duration following radiation for a spinal
tumor. Reconstruction was to be done
by staged transfer of a regional random (non-irradiated) flap. Integra was used not as the primary wound
closure but instead as an adjunct to the flaps. (Figure 17a) A large random flap from the non-irradiated
left lumbar area was elevated and transposed to cover the ulcer and part of
the dystrophic irradiated skin on the right.
Remaining dystrophic lumbar skin will be replaced when the base of the
current flap is elevated and moved, after the forward part of the flap is
healed. (Figure 17b) Integra was used to close the flap donor
site, opted in lieu of skin grafts to minimize pain, drainage, and nursing
requirements, and to limit skin grafts to only one subsequent procedure. Part of any good flap delay is to keep the
elevated pedicle from healing and revascularizing on its deep surface. Integra can also be seen buried underneath
the flap to prevent this. (Figure
17c) Fourteen days later, a delay
was done, consisting of division of the base of the flap, trying to force
more robust vascularization at the front end.
The Integra has a normal two-week appearance, still a transparent
window on underlying structures, but just starting to opacify from
histogenesis. The buried Integra
cannot be seen, but its presence under the flap continues to ensure flap
revascularization at the distal end rather then the middle or base. (Figure 17d) Three weeks later, the delayed flap is
healthy, uncomplicated, and ready for transposition. The Integra is fully regenerated and ready
for skin grafts. (Figure 17e) Taken at the same time as image 17d, this
image shows the back end of the flap, elevated and ready to move. The buried Integra is healthy. It has done its job, keeping the flap
unconnected in that area, making the flap easy and bloodless to elevate,
without creating new vascular stresses on the flap. The tail of the flap was moved across the
midline to replace the remaining dystrophic irradiated skin. Skin grafts were placed over both pieces of
regenerated Integra. (Figure 17f) A year later, everything is healed. The flap is healthy, Integra regenerated
skin is soft and compliant, and there has been no skin dystrophy nor
ulceration. All components of the
reconstruction healed without complication, and the problem was managed
entirely as an outpatient with little pain and no disability. Figure 17 a (top
left), b (top middle), c (top right), d (bottom left), e (bottom middle), f
(bottom right)
|
|
Case
Study 18, Arterial disease. A 64 year old man
with unreconstructable aortoiliac atherosclerosis developed toe ulcers. The patient went through progressive levels
of amputation, each complicated by further necrosis. (Figure 18a) Necrosis of the thigh amputation
illustrates a prime dilemma in operating on severely ischemic parts. Suturing wounds and flaps creates tension
which can diminish circulation. In the
presence of prior hypoperfusion, the reduction is enough to kill tissue. However, you also cannot reliably leave the
wound open, because highly ischemic tissues are intolerant of desiccation,
bioburden, inflammation, and injurious topical medicaments. This wound was sutured, and adjacent
tissues died. If it is now simply
debrided and left open, it will likely die some more. Most surgeons avoid this dilemma by simply
doing a higher level amputation, but in this case, that option has run
out. (Figure 18b) Integra solves this dilemma, allowing one
to debride the wound and then immediately close it without stress or tension
on the tissues. By arresting
inflammation, it controls yet another factor which threatens the ischemic
wound. Seen here two weeks after
debridement and Integra, the wound is healthy, and there is no necrosis at
any of the margins. Because surgical
revascularization was not possible, hyperbaric oxygen was used as an adjunct
therapy for two weeks after Integra placement. (Figure 18c) The Integra and skin grafts healed without
problems, and the reconstruction remained stable. Figure 18, a
(left), b (middle), c (right)
|
|
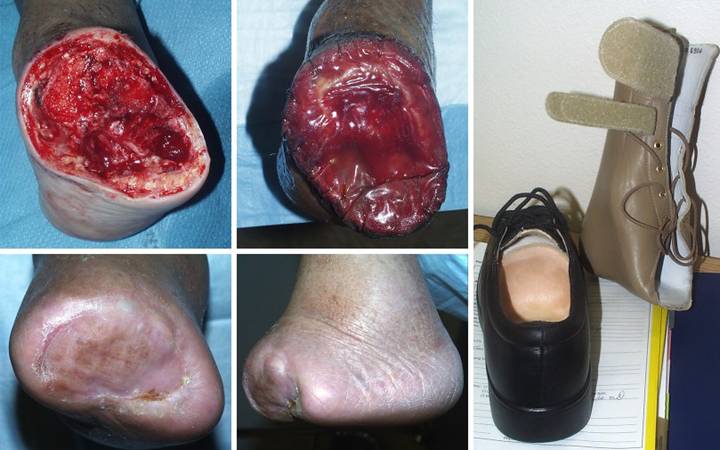
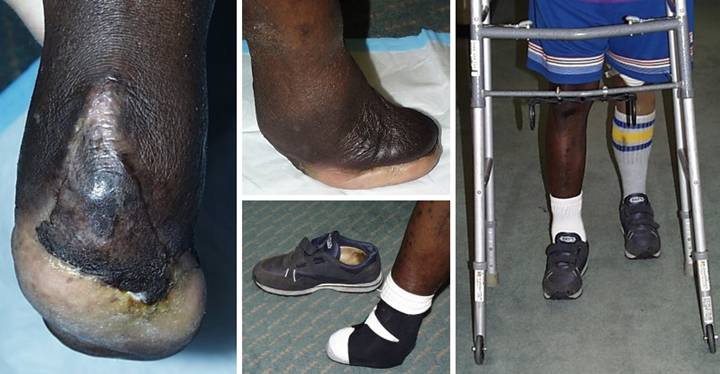
Case
Study 19, Arterial disease and diabetes. A 74 year old
man with diabetic atherosclerosis developed forefoot ulceration leading to
necrosis and abscess. (Figure 19a) Transtarsal amputation was done through
cuboid and cuneiforms. This is a
competent amputation because all major tendons to the ankle are still
inserted, and the ankle is motored and stable. However, osteotomies and intertarsal joints
are exposed and require cover. (Figure
19b) There is insufficient skin
for closure, but “creating” enough skin by further bone recession will detach
tendons and destabilize the ankle warranting below knee amputation. There are no local flaps, and arterial
disease precludes a free flap. Integra
is the simple, safe, and reliable solution.
(Figure 19c) The Integra
healed without problems. All care was
managed as an outpatient. By using a
posterior “wedge shoe”, the patient remained ambulatory during latter parts
of the reconstruction. (Figure 19d) This lateral view of the foot demonstrates
active dorsiflexion through the tibialis anterior tendon, confirming that
major tendons remain inserted and active.
(Figure 19e) Using an
insert at the front of a regular shoe, and a thin ankle-foot orthosis for
some additional stability, this patient has led an otherwise normal life. Two
years later, he remains completely ambulatory and active. Integra has been consistently successful in
closing and salvaging midfoot amputations.
There should no longer be any need to throw away an extremity only for
the want of a good flap. Integra
should be the preferred option for salvaging complex foot wounds in high risk
patients. Figure 19 a (top
left), b (top middle), c (bottom left), d (bottom middle), e (right)
|
|
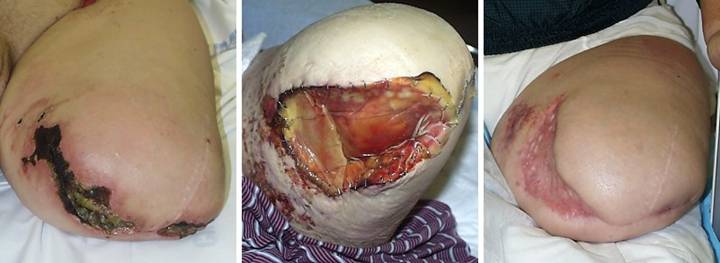
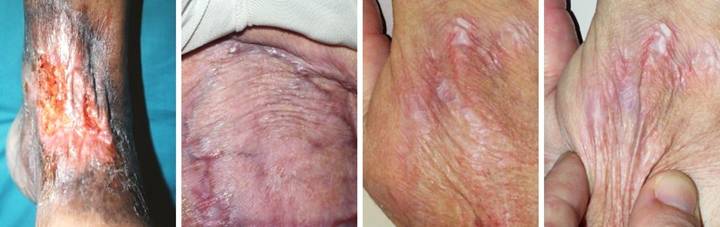
Case
Study 20, Select problem, achilles. A 44 year old
woman had a spontaneous achilles tendon rupture. Several failed operations resulted in
multiple wound complications and necrosis of the tendon. The area was eventually closed with a
rectus abdominis muscle free flap and skin grafts. (Figure 20a) The patient presented for consultation at
two years because the old flap and grafts were chronically dysplastic, with
recurring ulceration, pain, and dysfunction.
The graft and scar dysplasia were attributed to local
flexion-extension mechanics, and an attempt was made to excise and revise
them. This resulted in acute skin
necrosis and new ulceration. This
overall history is pathognomonic of an underlying immune or thrombotic
disorder. Along with a history of
retinal artery thrombosis, laboratory workup confirmed a hypercoagulable
antiphospholipid antibody syndrome. (Figure
20b) Closure of this wound has
challenges which defy conventional surgery, as already proven by her
history. Any incision risks more
necrosis and ulceration. A free flap
already failed, and more donor sites are unjustifiable. New skin grafts will develop the same
dystrophy and ulceration. The
reconstruction must be mechanically compliant and thin enough to accommodate
normal footwear. Integra overcomes
these obstacles with complete safety.
Wound debridement and warfarin anticoagulation were followed by
Integra. A second piece of Integra was
placed after the first one regenerated, in order to get a thicker neodermis
in this area of significant mechanical load.
There were no adverse events. (Figure
20c) The area healed without any of
the pathological changes that affected the original skin grafts, seen here at
one year. The reconstructed skin is
thin, soft, and compliant. It has
developed the transverse dermal creases that are expected in this area. Underlying old rectus muscle had been
sculpted to shape and preserved, and with subsequent therapy and activities,
applied load induced tendinous metaplasia, and the patient now has normal
ankle mechanics and normal function. Figure 20, a
(left), b (middle), c (right)
|
|
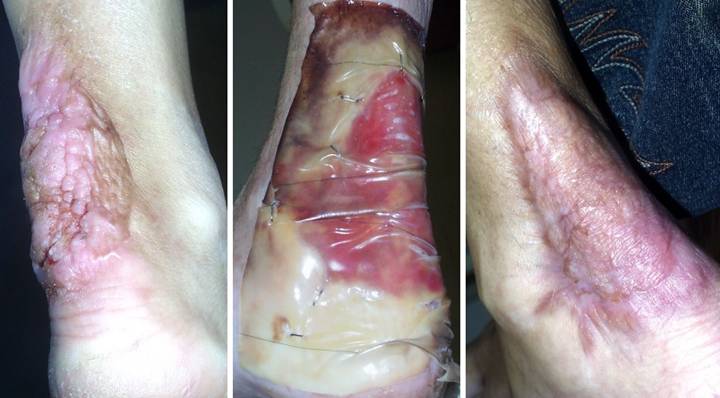
Case
Study 21, Select problem, heel. An 84 year old
woman developed multiple leg and foot ulcers related to diabetic vascular
disease and disabling senile illnesses.
Dependent on her family for physical assistance, foot preservation was
required so that she could stand and assist with wheelchair transfers. (Figure 21a) All ulcers were debrided and closed with
Integra, including large defects over the right achilles and heel. The extent of posterior calcanectomy can be
seen from the missing heel contour. (Figure
21b) A close up view of the heel
shows stable skin. Reconstruction was
uncomplicated, and all wounds healed.
Care was outpatient, and good results occurred with no risk to the
patient nor any dependence on living autogenous tissues which most likely
would have failed. Lifestyle was
preserved. Figure 21, a
(left), b (right)
|
|
Case Study 22, Select problem, stump salvage. A 53 year old man
had below knee amputation for diabetic vascular complications. (Figure 22a) Most surgeons seeing a stump complication
indiscriminately do an above knee amputation, most of which are
unnecessary. This wound had
granulation tissue and other wound module elements indicative of sufficient
circulation and competence to heal.
Necrosis was a consequence of avoidable technical factors. With patience, good wound care, and
suitable surgery, this below knee amputation can be preserved and
healed. (Figure 22b) After a period of debridement and good
daily care, the wound met criteria for reclosure. Note the exposed necrotic tibial surface
which needed excision. (Figure 22c) In surgery, the prepared wound was excised
and anterior tibia was decorticated.
For closure, the conventional choices of grafts and flaps were either
not available or not likely to work.
Had they been the only choices, higher amputation might have been
considered. However, Integra solved
this problem with no risk to the stump nor to the patient. Without Integra, there simply were no
reliable options. Integra is not an
alternative nor substitute for classic surgery, but rather an independent
surgical modality that works where conventional methods cannot. (Figure 22d) The stump healed. Throughout the reconstruction, a rigid
posterior platform splint maintained an extended knee, permitting expeditious
rehabilitation. This image is three
months after skin grafts. The patient
had fallen, causing a tangential avulsion laceration of the newly healed
epidermis. Treated and rehealed like
any similar laceration. this minor traumatic injury illustrates that Integra
requires some time to develop sufficient strength to bear up to the
requirements of daily life.
Appropriate caution and care should be taken for several months after
reconstruction. Figure 22, a
(left), b (second), c (third), d(right)
|
|
Case Study 23, Select problem, limb salvage. A 67 year old
woman developed foot necrosis due to complications of atherosclerosis. (Figure 23a) The foot was managed by basic topical care
and debridement. Operative
revascularization was done (saphenous vein graft to dorsalis pedis artery),
and the wound responded with rapid proliferation of granulation tissue. After that, even skin grafts would have
healed readily on the simple areas, but complex areas of exposed bones,
joints, tendons, and ligaments complicated the coverage issues. Flaps are conventionally needed, local or
free, but in an arteriopathic foot wound of this size, they are either not
available or have too many potential risks.
Integra bypasses all of these dilemmas. It solves the coverage issues with no
risk. (Figure 23b) The wound was debrided and closed with
Integra, shown here 6 weeks after placement and ready for skin grafts. (Figure 23c) The foot healed and remained stable, seen
here 6 months later. Figure 23, a
(left), b (middle), c (right)
|
|
Case
Study 24, Select problem, superiority to conventional
choices.
A 60 year old man with severe diabetes, vascular disease, and previous
left below knee amputation developed a large heel ulcer while hospitalized. He was offered, and refused, right leg
amputation. The issues of arterial
disease and anatomical location once again make conventional grafts and flaps
unworkable, but Integra reliably solves what conventional modalities
cannot. (Figure 24a) A large posterior calcanectomy was
performed. The osteotomy and achilles
insertion were closed with Integra, seen here as a face on view of the healed
reconstruction. (Figure 24b) His toes were also missing from previous
vascular problems, but enough foot remained to permit stable weight bearing,
stance, and gait. He is seen here in
full upright weight bearing on the healed foot. This profile view shows the extent of the
oblique calcanectomy, which merely followed the contours of skin
necrosis. (Figure 24c) For stability, the patient uses a space
filling orthotic wrapped around his ankle.
(Figure 24d) Using a
normal sneaker, his left leg prosthesis, and a walker, he is independent and
ambulatory. A complex problem was
solved with no donor sites, no risk to foot or patient, excellent biological
healing, and preservation of function and lifestyle, all as an
outpatient. For many of these
challenging problems, Integra is a superior option, working when and where conventional
repair, grafts, and flaps cannot. Figure 24 a (left),
b (middle top), c (middle bottom), d (right)
|
|
Case Study 25, Radiation ulcer, tissue engineering. An 82 year old
woman had a skin cancer of the scalp treated by radiation, 6500 cGy,
resulting in a chronically ulcerated parietal cranium. Integra was used
as a carrier of mitotically competent cells that were first incubated in a
remote subcutaneous wound chamber. The
transplanted cells were responsible for the graft healing. Details are presented below in the
discussion about radiation ulcers (figure 30). See figure 30 below. |
Key Points. In all of these
cases, certain common concepts emerge which define the value and virtues of
Integra for chronic and pathological wounds.
It is only as good as the general care of the patient, and accurate
diagnosis and management of underlying disease is crucial. It is a high quality artificial skin, and
while this effect is more dramatic in large acute problems such as burns and
fasciitis, it serves to protect wounds and underlying structures, minimizing
symptoms and simplifying care. However,
it is more than just an effective skin substitute. Its ability to suppress inflammation means
that it stabilizes pathological wound behavior and eliminates unexpected
exaggerated adverse wound complications after trauma and surgery
(pathergy). This permits safe
debridement and wound closure in the face of severe immunopathy and
ischemia. Because it is not living, it
will perform well where skin grafts cannot.
Its ability to get good results without donor sites nor any significant
“cutting and sewing” other than wound excision makes it extremely safe for high
risk limbs, patients, ulcers, and diseases.
Integra closes complex wounds with
exposure of visceral and musculoskeletal structures. In this regard, it serves many of the roles
that conventional flaps do. This means
it is the best solution when flaps are not available or cannot be used. There are times when it is simply superior to
flaps, permitting limb salvage and wound closure under circumstances where
conventional methods are technically impossible, guaranteed to fail, or apt to
create more problems than they solve. It
is effective for closure of bone, joint, tendons, visceral organs, and even
alloplastic hardware. For select areas
and problems, such as heel and achilles, midfoot amputations, and dorsum of the
hand and wrist, it is easier, safer, and gives better results than customary
methods. It is effective for a broad
spectrum of wound and soft tissue pathologies, often being the only successful
choice for arteriopathic, hematological, and immunopathic ulcers.
When conventional flaps are more
appropriate, Integra can be a useful ally, facilitating or simplifying the
overall reconstruction. Integra need not
be used only according to its nominal pathway of “one piece – healed”. Multiple sequential pieces for deliberate
purposes, and secondary touchup pieces to finish an otherwise largely complete
reconstruction should all be considered as part of the ordinary modalities of
its use. Integra often succeeds where
all else has failed, where no conventional options exist or they have run out,
and where patients and physicians have forgone hope. Integra seems to be resistant to recurrent
disease. It reconstructs a skin that has
superior mechanics and esthetics compared to scar, approaching the properties
of normal skin.
It simplifies care. It minimizes pain and nursing. It facilitates recuperation and preservation of function and lifestyle. It allows complex problems to be managed as an outpatient. Even when it is not fully healed, open Integra is always superior to the original wound. As such, patients are accepting of incomplete results, and they are willing to bear the time required to complete the reconstruction. Its effectiveness and safety profile can make it a preferred choice even when conventional methods would work. As a method of in situ tissue engineering, it is not a substitute nor alternative to flaps and grafts, but rather a new paradigm of surgical wound repair with its own distinctive role. This role is especially effective for treating chronic and pathological wounds.
DISCUSSION
General
Discussion
Methods of wound closure, from
topical care in support of natural contraction through surgical repair, flaps,
and grafts, are usually opted by individual circumstances. These include size, location, acuity or
severity of the wound, exposure of visceral or skeletal structures, and patient
age, risk, and comorbidities. Most
surgery and wound care are done with the implicit faith that wound healing is
competent and that the wound or repair will heal. However, these assumptions are invalidated by
certain chronic illnesses which cause chronic ulceration and impair the
physiological process of wound healing.
Arterial insufficiency, venous disease, immunopathies,
hematopathologies, and other disorders are the causes of chronic refractory
ulcers which defy usual attempts to close them.
Integra Dermal Regeneration Template®, can reliably
close such ulcers.
The 111 patients in this study all
had chronic and pathological ulcers.
Most had failed multiple modalities of treatment. One third had prior failed operations. Many were deemed hopeless by other
physicians, and some had been offered amputation. For most, disease and pathological anatomy
made them ineligible for the repairs or reconstructions that would have been
done for comparable defects due to trauma in healthy patients. Part of the good outcomes in most of these
patients can be attributed to systematic and comprehensive wound management,
including proper diagnosis, treatment of underlying diseases, proper wound
preparation, and long term management by a consistent and knowledgeable staff
of physicians and allied health professionals.
Nevertheless, for most of these patients, Integra was the crucial
component of care which solved the problems.
Integra closed wounds when pathology could not be fully eliminated, when
wound healing was delayed or impaired, and when flaps or other complex repairs
ordinarily would have been required but were disallowed by circumstances of
disease or anatomy. It did so safely,
without donor sites nor significant risk to any patient.
The author’s practice is devoted
exclusively to wounds and reconstructive surgery. In the 6 years of this study, the 132
patients having Integra were a small fraction of the entire operative and
clinical experience. Integra was not
used indiscriminately, neither for its novelty nor any other reason. For example, for each venous patient treated
with Integra, there were many more treated by compression, topical modalities,
phlebectomy, skin grafts, and other care.
All patients were treated according to some disciplined schema for the
evaluation and treatment of chronic wounds and their underlying causes, with
Integra opted based upon certain consistent criteria. To understand the indications for Integra for
chronic and pathological wounds, its unique biological properties must be
understood.
Properties of
Integra
General Biological Effects
Integra is a non-living semi-biological implant. Both the native and the regenerated material have desirable properties. In its role as an artificial skin, the Integra sponge persuades the wound that there is no injury, suppressing inflammation and its sequelae while the silicone functions as an effective epidermis. In its role as an agent of regeneration, Integra has histoinductive and histoconductive effects on mesenchymal tissues which lead to a regenerated analogue of normal dermis. Integra’s favorable properties, its clinical utility, and its superiority for certain reconstructions all derive from its structure and composition.
Structure
Chemical composition. The Integra
material is made from type 1 collagen (from bovine achilles tendon) and
chondroitin-6-sulfate (chondroitin sulfate C, a glycosaminoglycan, GAG, from
shark cartilage). These materials are chemically cross linked then
processed into a porous sponge referred to as CGM, collagen-GAG matrix.
Unlike many proprietary collagen dressings marketed for wound care, Integra is emphatically
not a “collagen product”. Integra depends on both components.
Collagen provides mainly structural form. The chondroitin, 8% of weight,
is what confers key properties. The glycosaminoglycans, including
hyaluronan, dermatan, keratan, and others have vital roles in constituting the
extracellular matrix and regulating cell development and differentiation. They predominate in un-proteinized embryonic
tissues, and they accumulate in fetal wounds which heal by regeneration without
inflammation nor fibrous scar 8. 9, 10. Integra’s chondroitin also masks binding
sites on the collagen, thereby preventing platelet adhesion and resulting inflammation 11,
12.
Microarchitecture. The average pore
or cell size of the spongy manufactured material is 5 – 150 microns, averaging
80 – 100 microns. This size was
deliberately engineered. Too small, and
histogenetic cells can not invade nor occupy the matrix. Too capacious, and potential histogenetic
cells would “see” a non-stimulatory flat surface. This size, which tends to match the collagen
reticulum in normal dermis, is within a target range that engages cells to
undergo histogenetic proliferation.
Macroarchitecture. The spongy Integra
matrix is formed into sheets
Acute Physiological Effects
Suppresses inflammation and
its effects. Inflammation is the normal response to
injury, leading to normal inflammatory fibrous wound repair. When Integra is applied to a wound,
inflammation ceases. Not only does it
seem to be “invisible” to platelets and inflammatory leukocytes, but it also
seems to be recognized as self. At no
time are there microscopic inflammatory cell infiltrates nor any clinical signs
of inflammation. Pain is often
conspicuously absent after Integra, and any pre-operative periwound erythema and
edema abate rapidly. Hypotheses
explaining this phenomenon include: (1)
lack of platelet adhesion prevents the thrombotic cascade to inflammation from
being triggered (figure 25); (2) the
artificial skin sequesters the wound, eliminating ambient exposure,
desiccation, bioburden, and similar secondary injury; (3) the chondroitin matrix looks sufficiently
like normal tissue that blood borne leukocytes and lymphoid cells that might
find their way into the matrix do not recognize anything abnormal that would
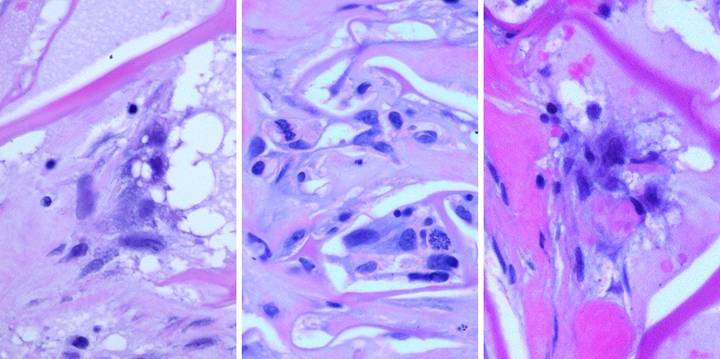
trigger a defensive response 13 (figure 26).
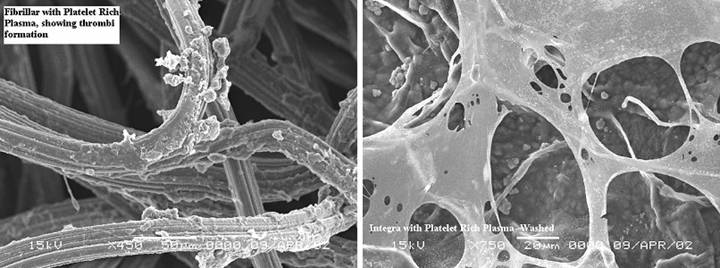
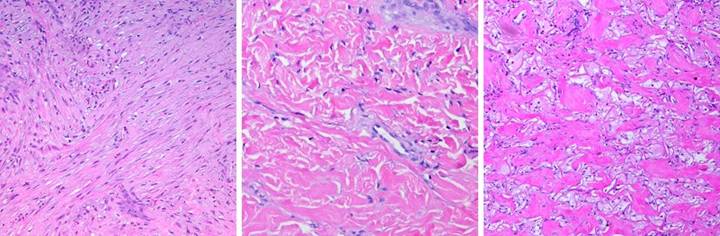
|
Figure
25, a (left), b (right) (a) Platelets adhere
as expected to a collagen-cellulose matrix that has been incubated in
platelet rich plasma. (b) Under the same
circumstances, platelets do not adhere to the Integra collagen-GAG
matrix. The chondroitin has rendered
the collagen invisible to platelets.
(Photographs on file, Ethicon, |
|
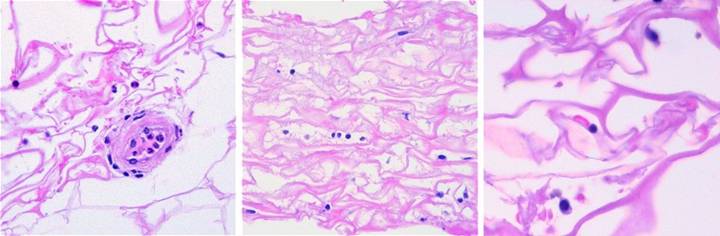
Figure
26, a (left), b (middle), c (right) (a) This biopsy was
taken 4 hours after excising skin and placing Integra for an elective
reconstruction with no prior inflammation.
A blood vessel is present at the wound surface between Integra matrix
(top and left) and normal adipose (bottom and right). There is margination and migration of
polymorphonuclear leukocytes (neutrophils).
This means that post-traumatic thrombosis and platelet effects have
recognized the injury. This is the
normal response to injury, the start of inflammation. (b) For whatever
leukocytes that do find their way into the matrix, they find nothing
exciting. They do not recruit other
cells nor incite any other component of inflammation. They subside and disappear, and any
incipient inflammation that would accompany a normal injury is completely
extinguished. Seen here at 5 days
after surgery and Integra, cellularity is sparse, with neither neutrophils,
plasma cells, eosinophils, lymphocytes, nor monocyte-macrophages. At no time does a defensive response ever
appear in the matrix. Note the cells
that are present. These are the early
histogenetic cells. The small round
cells are the “pioneers”. Some of them
have elongated and flattened into “transitional cells”, a sign of recognition
and attachment to the matrix, a characteristic interaction of cells with glycosaminoglycans. (c) This biopsy is 4
days after Integra, from another patient.
The situation is the same, no inflammation. Three cells are seen. The small mononuclear
lymphoid cell (but not a lymphocyte) at the center is an early pioneer. These seem to be “patrol cells”, either
resident in tissues or blood borne, which find the matrix by
happenstance. Defensive reactions and
cellular recruitment do not occur. The
round cell at the bottom is a pioneer which is starting to accumulate
cytoplasm, the first sign of matrix recognition. The cell at the top is starting to flatten
and elongate, denoting attachment to the matrix. Once this transition happens, histogenesis
begins. Histogenesis could not occur
if inflammation was active. The
effects of Integra re two: it quenches
incipient inflammation triggered by injury, and it does not incite any
inflammation on its own. It is
recognized by the host as an acellular “self”. |
Suppression of pathergy. General pathergy
(in its more liberal contemporary definition) refers to self-destructive
effects of injury and inflammation which cause necrosis, tissue lysis, wound
bursitis, dehiscence, and other undesirable wound complications. Paradigms are the injury-induced necrosis of
pyoderma gangrenosum and the dermatitis of Behçet’s syndrome. It occurs with any disorder causing severe
ischemia or pathological inflammation including athero- and other
macro-occlusive diseases, hypercoagulable, microthrombotic, and micro-occlusive
disorders, autoimmune vasculitis and angiopathies, and any active immunopathy
or connective tissue disorder or similar disease of immunity and
inflammation. In these disorders, every
surgical procedure from simple debridement and biopsy to amputation and complex
repair is at risk. Applied to such
wounds or patients, Integra controls or eliminates this risk. By suppressing inflammation, by appearing as
normal tissue, and by sequestering mesenchyme, it arrests the progressive
auto-amplifying injury which leads to acute wound failure.
Immediate closure of wound
and recognition as normal tissue. The
biocompatible sponge and the silicone pseudo-epidermis together form an
effective artificial skin. When Integra
is placed on a wound, all of the events which define the usual response to injury
are halted. Physiologically, the wound
ceases to be a wound. To the lymphoid
patrol cells which do eventually find the matrix, the chondroitin lattice
appears to be an acellular but otherwise normal tissue. The only response triggered is a regenerative
one.
Sub-Acute Physiological
Effects
Suppresses normal
inflammatory wound repair. Inflammation
begets normal wound repair. Macrophages
(transformed monocytes) accumulate in a wound and direct repair via cytokine
stimulation of local histoprogenitor cells.
This leads to the proliferative wound module of inflammatory repair
which produces scar 14. By
turning off inflammation, Integra turns off this entire series of events. Integra heals by a different mechanism. Its histogenesis does produce collagen, but
it is comparable to normal dermis and distinct from scar. Assuming that the Integra remains uninjured
and uncomplicated and that no inflammation is thereby incited, inflammatory
fibroplasia (scar) never occurs. This
means that contractures, keloids, and other reactive or pathological fibroses
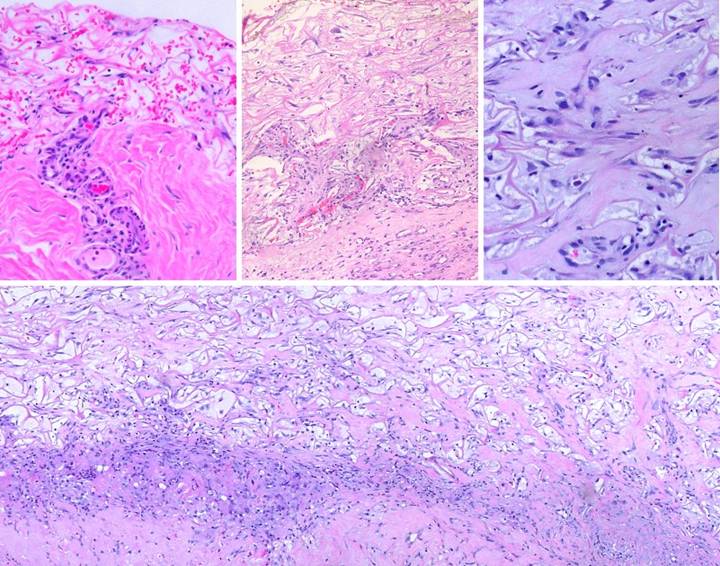
and their clinical effects are averted (figures 27, 28-6,7,8).
|
Figure
27, a (left), b (right) (a) These two images
contrast normal inflammatory wound module repair versus Integra
histogenesis. In this image, Integra
(on the thigh following necrotizing fasciitis) is regenerated and ready for
skin grafts. In an open seam between
two pieces of Integra, normal wound healing has occurred, resulting in
granulation tissue in the gap. (b) This is healed Integra
at 24 months (on the flank in a 7 year old girl). Looking past the epidermal pigment
variegation and the contour depression from lack of subcutaneous adipose, the
Integra skin per se looks mostly normal, soft, pliable, free of erythema and
fibrosis. In the center though is
typical hypertrophic scar, arising where normal wound healing and granulation
tissue developed in a seam gap. The
differences in these photographs, between granulation tissue and Integra
histogenesis, between scar and Integra neodermis, epitomize all of the
biological differences between these two processes. Histological examination of the process, as
shown in the next series of images, illustrates the biophysics behind these
differences. |
Induction of embryonic
histogenesis. If
Integra did nothing other than control inflammation, pathergy, and scar, it
would still be a valuable device, but it would then be just another biological
dressing ultimately needing replacement by autogenous grafts. What makes Integra unique among all other
surgical grafts and implants is that Integra histogenesis is highly analogous
to normal embryonic dermatogenesis. The
surgeon who uses Integra is incubating a re-engineered tissue devoid of scar
and having the characteristics of developmentally normal dermis. The geometry and especially the aminoglycans
of the matrix are presumed to be the key triggers for this phenomenon. The similarity of embryonic and Integra dermatogenesis,
and their distinction from inflammatory healing and scar can be understood by
observing the histology of these events, depicted in the figure 28 sidebar.
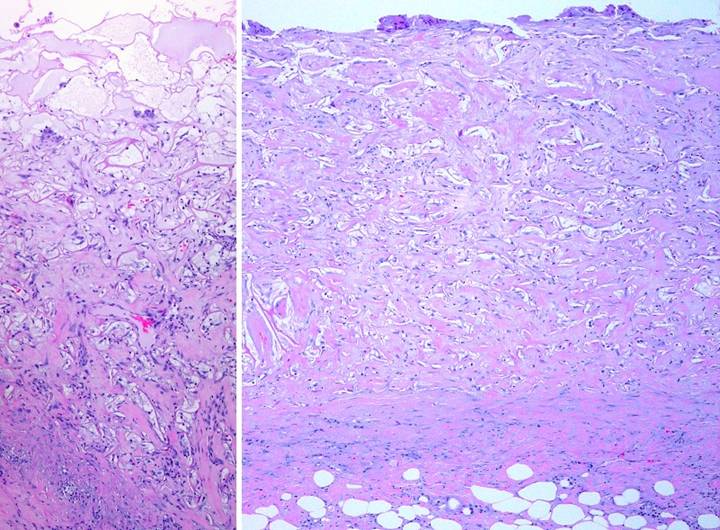
|
Figure 28 sidebar, the process of Integra histogenesis. Integra artificial skin has beneficial physiological
effects when applied to acute and pathological wounds. Regenerated Integra neodermis has superior
anatomical and functional characteristics that obviate the need for late
surgical revision of scars and contractures.
There are anatomical and biophysical reasons why Integra has these
properties Figures 28-1 through 28-8
document the process of Integra histogenesis.
This series of images and legends are a sidebar to the main subject of
this report, but they are included so that some of the reasons for Integra’s
favorable performance can be seen directly.
Figure
28-1, transformation of syncytial
cells and early “first set” histogenesis,
a (left), b (middle), c (right) Figure
26 showed suppression of inflammation.
This was followed by early population of the matrix with a low density
of small pioneer cells which then attached to the matrix. Matrix attachment is the crucial
transition, triggering these cells to perform a latent function that has no
parallels in normal post-fetal life.
They are about to undergo a process completely analogous to embryonic
dermatogenesis. The geometry and gross
anatomy of the embryonic and Integra systems are different, an enlarging
solid tissue model versus space filling of a fixed void volume, but the
dynamics of cells and their interactions are the same. The earliest histogenetic events are shown
in this set of images. (a) At 13 days, the
matrix remains only sparsely populated.
Small pioneers and transitional cells can be seen, but there are also
large cells. Occurring as singlets or
in small clusters, these polymorphic cells with abundant basophilic cytoplasm
and nucleoplasm are the “syncytial fibroblasts”. They are what the pioneer cells have
ultimately transitioned to. From
bottom to top, host tissue to silicone, they are distributed evenly
throughout the matrix. They will
multiply into small independent insular clusters of cells which begin to make
fine fibrillar collagen. This is the
“first set” of histogenesis. So far, this developing tissue has no vascular
infiltration, so first set histogenesis will be limited by substrate
diffusion. The fact that early pioneer
cells and syncytial clusters are scattered uniformly through the matrix, at
seemingly long distances from the host, is not surprising. Cell-to-vessel distances are vitally
important to histogenesis, gas exchange, substrate supply, and
vasculogenesis. However, the
biophysics of these processes dictate that “cell-to-vessel distance” is a
normalized metric measured in unit cell widths along a diffusion gradient, and
not in actual physical lengths. As long
as there are only about 5 – 10 cells on a diffusion vector, they can be
widely scattered. Thus the matrix is
uniformly dispersed with early pioneer cells cum syncytial fibroblasts, and
they all function normally. However,
as they become metabolically active (thereby lowering the system’s threshold
cell-to-vessel distance), and as the cells start to divide and form clusters
with more cells, new blood vessels are required to restore effective vascular
density. Until vascular supply is
established, continued growth to confluence and filling of the space will be
delayed. Like all embryonic and other proliferative cells, these
burgeoning syncytial fibroblasts are starting to make angiogenic factors
which, via their effect on nearby angiocytes, will attract new vessels. In the substrate fascia on which the
Integra sits, the blood vessel to the left is normal, with small flattened
orderly angiocytes. On the right side,
close to the matrix, angiocytes have become large, polymorphic, mitotic (not
captured in this image), and migratory.
Blood vessels are the normal reservoir of proliferative mesodermal
cells which can heal a wound or regenerate tissue. Under the stimulus of the proliferating
syncytial cells, the angiocytes are in turn “coming to life”. (b) This is a closer
view of a syncytial cluster. The name
is taken from descriptions of embryonic dermatoblasts which appear identical
to the Integra cells: large,
polymorphic, indistinct, with numerous pseudopods 15. Their boundaries cannot be seen clearly,
hence “syncytial”. The cluster is
composed of less than a dozen cells, within which faint pink young collagen
is starting to form. Note that this
cluster lives and functions in physical isolation from any other biological
structure. Further growth and proteogenesis
will be limited by competition with other clusters until vasculogenesis
starts to occur. (c) In the center is a
pair of two indistinguishable cells accompanied by some pale pink material,
early fibrillar collagen. Nearby,
spindle shaped migratory cells recruited from the substrate are beginning to
invade the matrix, and organization of these cells into a new vessel
penetrating the matrix is seen at right.
This is the inception of a tissue level of histological organization.
Figure
28-2, functions of the syncytial
fibroblasts, a (left), b (middle), c
(right) The
syncytial fibroblasts are of crucial importance. Their appearance is the keystone event in
Integra histogenesis. They are the
transition between normal cells and embryonoid processes. They originate and regulate the
histogenetic process within the matrix.
They are not normal cells in post-fetal life, and they never appear
during normal post-inflammatory wound module healing, neither as they appear
here nor in any comparable form. Their
morphology and function are typical of embryonic cell interactions with
glycosaminoglycans. The Integra matrix
is explicitly triggering this transformation and histogenesis. (a) A close-up view a
highly proliferated, metabolically active cluster that is not yet
vascularized. Cells are large,
indistinct, basophilic and granular, and metabolically and proteogenically
productive. Young collagen is
abundant, within the cluster and to the left.
The cluster can get no larger until nearby vascularization
occurs. (b) Once an active
domain is vascularized, a “second set” of histogenesis occurs. The clusters can then grow until space is
filled and loss of contact is corrected.
Cells and collagen accumulate, and the many independent domains of histogenesis
grow to confluence, and in so doing they create an organized tissue. In this view, second set histogenesis is well
underway. Cell density is increased
and pink collagen is more abundant. In
some areas, the collagen is turning fibrous, from pale to a denser more
saturated pink, and associated cells are getting trapped and flattened in
that new collagen. New cells come from
two sources. Some are migratory
histogenetic cells from underlying blood vessels, and some come from mitosis
of the embryonoid syncytial cells, two of which are captured here in
prometaphase and metaphase. (c) In this cluster, large syncytial cells in the center
continue making young fibrillar collagen.
At the periphery, fibrous collagen is getting dense, taking on an organized
lamellar architecture, with entrapped cells now looking like classic
fibroblasts. Compared to normal
inflammatory healing and scar, this tissue is relatively acellular, and the
collagen is wavier with interstitial spaces.
Figure
28-3, vasculogenesis and “second set”
histogenesis, a (left), b (middle), c
(right), d(bottom) At 10
– 20 days in normal wounds, fibrous repair is sufficiently mature to permit
suture removal. Skin grafts are
adhered and vascularized (or else they have died by now). But in Integra, the process of histogenesis
is just barely underway. The early
period of pioneers, syncytial clusters, and incipient angiogenesis was
organized at a cellular level. The
system now moves into the second set of development, where true histogenesis
occurs, the formation of a confluent structurally integrated tissue. (a) In this view,
scattered syncytial clusters are exerting their effect on subjacent blood
vessels which are responding. Vascular
invasion of the graft is beginning, and as a circulatory network is
established, cell proliferation and connective protein production proceed. (b) Second set
angiogenesis can only occur in the vicinity of new blood vessels. Tissue formation begins around vascular
entry points, and the process pushes further into the matrix following the
paths of vasculogenesis. In this view,
a local domain of vascular ingrowth is associated with dense filling of the
matrix. Beyond this consolidated
domain, insular syncytial cells in empty matrix are creating the angiogenic
cytokines which will attract new vessels to reach them. (c) In this close-up
view, the cellular events of second set histogenesis can all be seen. Some pores are empty, and some are filling
and growing to confluence. Syncytial
cells are making collagen, and some cells are entrapped in collagen, assuming
a common fibroblast appearance.
Clusters of migratory spindle cells are angioblasts reorganizing new
blood vessels. (d) A wide view shows
that the process occurs concurrently in many locales, and the locales
gradually become confluent. A true
histological and physical connection of matrix to host is occurring.
Figure
28-4, inflammatory angiogenesis versus embryonic vasculogenesis, a (left), b (second), c (third), d(right) Angiogenesis
is obviously crucial for basic cell survival and tissue growth. However, seen from a systems or physics
point of view, vasculogenesis and vascular network topology simply reflect
the dynamical growth of the host tissue.
In normal embryogenesis, vascular network formation is a tightly
controlled closed loop process. The
same is true of Integra vasculogenesis, whereas inflammatory repair has
unregulated open loop angiogenesis. Vascular
density is thus another indication of Integra’s identity to embryonic growth
and its distinction from inflammatory repair 16, 17, 18. (a) Inflammatory
angiogenesis is an open loop process.
Macrophages which stimulate and attract angioblasts are extrinsic to
the developing tissue, and they are not inhibited by the arrival of vessels
(the same is true for histioblasts and fibrogenesis). Compared to normal tissues, granulation
tissue is hypervascular due to robust open loop overproduction of blood
vessels. This is seen grossly as a
saturated red color that contrasts sharply with pale surrounding normal
skin. (b) Histology
corroborates gross appearance. Blood vessels are excessively dense and
enlarged, far in excess of what is needed for basic blood supply and normal
metabolic needs. After the wound is
closed and can mature, vascular density slowly returns to normal over months
or years. (c) This is a lower
power wider view of regenerated Integra, yet there are far fewer vessels than
in the picture of granulation tissue.
Recognizable as thin cellular chords and small transverse rings,
vessel count and blood volume are much less.
The tissue is obviously alive, so vascular density is sufficient. It is in fact exactly matched to the needs
of the tissue, because Integra vasculogenesis is a closed loop interaction
between syncytial cells and angioblasts. (d) Integra in this picture is fully regenerated and
ready for skin grafts. Its white color
appears virtually identical to the adjacent normal skin. This is
because they have equal vascular density, both densities being just exactly
what is needed to supply the tissue. Precise and efficient vascular
network formation results because Integra and embryonic vasculogenesis are
nearly identical dynamical processes.
They are both tightly regulated closed loops controlled by cells
(syncytial fibroblasts and embryonic dermatoblasts) that are intrinsic to the
developing tissue. Unlike what happens in scar, vascular density in
Integra is just what it should be, and it will not change as the tissue
matures.
Figure
28-5, confluent histogenesis and
matrix filling, a (left), b (right) (a) Although first set
syncytial histogenesis begins with cells scattered evenly throughout the
matrix, second set histogenesis is limited by the availability of new blood
vessels. Of necessity, these can only
enter at the base and progress upward.
Therefore, organized confluent tissue formation also begins at the
base, rising toward the silicone. This
17 day image shows regeneration half complete, 1-2 weeks away from skin
grafting. At the top level, empty pores have scattered pioneer and
transitional cells. Just below, syncytial clusters bide their time,
awaiting the arrival of nutrient capillaries, making some fibrillar collagen
as they wait. At center level, migratory angioblasts are organizing
into early erythrocyte-carrying vessels. Cell and collagen density are
increasing, but the central layer is still more basophilic than eosinophilic,
more cellular and cytoplasmic than proteinized. In lower strata, large
conducting vessels carry blood locally and to developing strata above, and
bright pink dense fibrous collagen is becoming the bulk of the tissue.
At the lowest level, entrapped fibroblasts are flattened between maturing
parallel collagen bundles. Matrix and host now have a firm physical
connection of collagen and vascular structures. Blood vessels in the
substrate fascia are still hypertrophied, but basophilia is more cytoplasmic
than nuclear, the proliferative response winding down as active histogenesis
and angiogenesis shift to upper levels where mid-zone vessels are now the
angiogenic source. This process will continue shifting upward. (b) As second set
histogenesis progresses upward, the result is a uniformly consolidated
matrix, seen here at 6 weeks, biopsy taken at the time of skin grafts.
Note the large foreign body giant cells at the top. Often appearing
within a week of Integra’s placement on the wound, this is a common and
normal response to the unnatural silicone.
Some people get them, some do not.
It is a safe, non-inflammatory process which at worst causes some
turbid blistering and premature silicone separation. This has no effect on the matrix, and it
does not jeopardize the skin grafts.
Figure
28-6, collagen morphology of Integra
versus dermis versus scar, a (left), b
(middle), c (right) The
similarities of Integra and normal skin, and their distinction from scar can
be seen histologically. (a) This is young
scar, representing normal inflammatory wound repair at peak fibroplasia. Highly cellular at this point, the densely
packed fibroblasts are making thick chords of collagen. In ensuing weeks, the scar will become even
more heavily collagenized. Increasing
connective proteins will make the scar progressively less compliant or
distensible. Dense packing of fibers
and lack of interstitial spaces and individualized bundles mean no fluidity
of the material. There is no folding,
coiling, or waviness that would confer elasticity or distensibility. Collagen bundles are multidirectional at
this point, but subjected to tensile stresses, they will reorient themselves
to resist that load. Compounded
further by myofibroblast mediated contraction, the scar becomes ever more
non-compliant. These are the
mechanisms of scar’s distinctive and detrimental properties, the origins of
contractures, stiffness, strictures, stenoses, and disfigurement. (b) This is normal
reticular dermis. Typical fibroblasts
are dispersed in typical densities, seeming quite dilute in comparison to
scar fibroblasts. Collagen bundles are
variable in size, mostly large, with great variability in orientation. They are highly individualized and
distinct, separated by interstitial spaces.
The bundles are also coiled, wavy, or springy. All of these factors permit elastic
compliance of the material, and normal motion of associated body parts (c) This
is fully regenerated Integra. Compared
to scar, it has a completely different histology, morphology, and
biomechanics. Cellularity
is low. Collagen bundles are thick but
organized into individualized bundles or packets. Collagen conforms to the matrix without
causing any distortion or contraction of the septae. The matrix itself partitions the collagen,
having a similar effect on the structure and mechanics of the material that
the interstitial spaces have in normal dermis. These spaces, the interruptions and
incoherence between collagen clusters, and the springiness of the septae mean
that the material is fluid, elastic, and deformable. The Integra does not look precisely like
normal dermis, but the two materials share many structural properties, and
Integra can be expected to behave like normal dermis. Both are distinctly different than scar
whose collagen density and geometry defy mechanical compliance.
Figure
28-7, composite structure of Integra
versus dermis versus scar, a (left), b
(second), c (third), d (fourth), e (right) This
is another direct comparison of Integra, dermis, and scar, looking not just
at collagen, but at the composite skin structure. (a) Papillary dermis
is a lamina propria that supplies metabolically active epidermis. Its generation and the papillation of the
epidermis are epidermal functions that are independent of the substrate. In this image, regenerated Integra has
recently been skin grafted. Epidermal
strata have reorganized. There is no
papillary dermis yet, but increasing subepidermal cellularity indicates the
onset of this process. (b) Integra at one
year. Epidermis is normal. The
papillary dermis is also completely normal, and it is a clearly defined
lamina distinct from the Integra which is the de facto reticular dermis. Even at this late interval, the matrix is
present, undistorted by scar contraction, maintaining the architectural
features that confer compliance and which make it similar to normal dermis
and distinct from scar. (c) For comparison,
this is normal skin, with distinct reticular and papillary layers, and all of
the architectural features which characterize normal skin and its physical
properties. (d) This is normal
healed scar. A thin papillary dermis
and subepidermal plexus have formed.
The true scar, substituting for the reticular dermis, continues to be
dense, cellular, architecturally antithetical to extensibility and mechanical
compliance. (e) This image is
healthy Integra at 4 years. It is
included to demonstrate that Integra continues looking like normal dermis,
never contracting, never accumulating scar, for prolonged periods. Much of the matrix itself is still present
(collagen-chondroitin cross linking is an essential part of Integra’s
chemical engineering design and manufacture, and it confers remarkable
stability and insolubility). There is
no evidence that Integra has any diffusible moieties nor any cytotactic
properties. The only mechanism for its
disappearance would seem to be slow passive hydrolysis, preserving much of
its original architecture for as long as four years. This also explains why Integra does not
degenerate, why it can safely wait over pathological wounds or voids or
alloplastic materials, surviving conditions that kill skin grafts.
Figure 28-8, the
wound module of inflammatory repair versus Integra histogenesis, a (left), b (right) This final comparison shows regenerating Integra side by
side with a proliferating wound module, and regenerated Integra side by side
with scar. (a) Just like normal
tissue, if Integra is injured, leaving an open wound, it will trigger
inflammatory repair. For this image, a 4 week Integra biopsy was left
open, resulting in a bead of granulation tissue, and then 2 weeks later this
overlapping biopsy was taken. The boundary shows an active normal wound
module arising from the Integra. Both systems are developing side by
side, each according to its own set of stimuli and responses. This is
the same individual, the same cell biology, the same genome, but cells are
behaving according to two vastly different programs. Injury has
triggered the inflammatory wound healing response which leads to scar. Integra has triggered the embryonic
histogenesis program. This response to
injury and surgery does not naturally happen in post-fetal life. (b) This specimen is
one year after Integra. On the right is properly healed Integra,
looking very much like normal dermis. Epidermis and the papillary
dermis are normal. On the left, Integra skin converges on an area of
normal post-inflammatory contracted scar. Scar is not static, and is it
matures over long intervals, collagen remodeling allows scar to begin
resembling dermal collagen, as is the case here. However, seen juxtaposed
against the Integra, one can appreciate why Integra behaves so much more like
normal skin. |
Related Therapeutic Effects
Semibiological, but not
alive to begin with. “Biological dressings” are used to protect
wounds, control inflammation, prevent pathergy and complications, and promote
wound repair prior to definitive closure.
Cadaver allograft, porcine xenograft, allogeneic amnion, and
non-biological materials (e.g. Biobrane®, Bertek Pharmaceuticals,
Inc.,
Biological superdressing. As a non-living
semi-biological material, Integra is an excellent biological dressing, but it
has an added advantage. As the Integra
covered wound becomes healthy and competent to proliferate new tissue, the
Integra then acts as the matrix for new tissue growth. Thus, the Integra does double duty, first as
a non-living biological dressing which does not die nor degenerate, and then as
the agent of skin regeneration and reconstruction. As such, it is much more than just a simple
or passive biological dressing, and the term “biological superdressing”
designates its ability to automatically transition into the mode of active
histogenesis and skin regeneration.
Histoconduction and bridging. The Integra sponge is a trellis that guides
the ingrowth of new tissue. When Integra
is placed on a healthy wound, histioblasts migrate into the material from the
base of the wound, dragging angioblasts in their wake, and then creating a
living tissue. It is customary to think
of this ingrowth as an orthogonal function, the direction of growth being
vertical, at right angles to the wound surface, the same process taking place
concurrently at each infinitesimal area of the wound. But there is nothing which prevents
histioblasts from migrating tangentially.
If there is a gap or void in the wound which cannot source cells into
the overlying Integra, cells will migrate in from surrounding healthy
regeneration-competent areas. This
property is analogous to other histoconductive matrices such as cancellous bone
grafts. Tangential histoconduction means
that Integra regeneration can “bridge” over surfaces that cannot heal. Objects which customarily need flaps for
closure can instead be healed with Integra.
This includes, tendons, open joints, cartilage, and even alloplastic
hardware.
Suppression of scar,
avoidance of scar sequelae. Scar is
the product of inflammatory wound healing.
It is a dense disorganized deposition of collagen distinctly different
than normal dermis and fascias. An indiscriminate glue that binds injured
tissues together, scar ensures the health of subjects after injury and surgery,
but it also has a dark side. The cause
of deformities, contractures, strictures, stenoses, and similar sequelae, it is
one of the most prevalent causes of long term morbidity and dysfunction and the
need for corrective surgery and physical therapies. Because Integra stops
inflammation and normal repair, it stops scar formation. No scar means none
of its complications. It can control fibroplasia where old scars once
were, it can prevent new scars, and it can correct the adverse consequences of
scar. It even suppresses or prevents pathological scars such as keloids
(figure 29).
|
Figure
29, a (left), b (second), c (third),
d(right) (a) Contraction and
non-compliance of scar causes the common clinical problem of contractures
across joints. In this case, the scar
is on the dorsal ankle after an old burn.
Motion of the ankle puts tension on the scar, causing it to undergo
tendinous metaplasia, further decreasing compliance. Motion (from normal walking) also fractures
the scar, causing ulceration which begets more scar. This image not only illustrates the nature
of chronic scar and contracture, it also highlights precisely the kind of
case which is reconstructable with Integra. (b) Integra is soft,
pliable, and compliant, comparable to normal dermis. While nearby scars were still red and
stiff, this recent Integra on the back was very deformable, wrinkling and
folding normally in response to any motion or force. (c) In another
patient, the dorsum of the hand was reconstructed with Integra after
granulomatous ulceration. The new skin
is soft and pliable and has normal dermal wrinkles. (d) In the same
patient, on the dorsum of the hand, manipulation of the material confirms its
normal properties. These properties
allow joints, the face, and other mobile parts to be reconstructed without
contractures. |
Similarity to normal dermis,
favorable mechanics. More
than just taming scar, regenerated Integra has properties comparable to normal
dermis. Already apparent from histology and histogenesis (figure 28
sidebar), these attributes are confirmed by mechanical testing of these
materials 19. The real proof though is “in the pudding”.
Clinically, its texture and quality, its suppleness and pliability, its
compliance and elasticity confirm its distinction from scar and its similarity
to normal skin (cases 15, 20). This means that the regenerated skin is
not apt to contract nor cause the clinical sequelae of scar. There have been numerous independent
observations that, compared to skin grafts:
(1) healed Integra is better, comparable to normal skin 20 - 24;
(2) Integra mechanics and physical properties avoid contractures and give
superior functional results and improved range of motion 20 - 22, 2 5- 32;
and (3) cosmetic appearance is also superior 20, 22, 28-30, 33 - 39.
Local soft tissue pathology
is controlled. Integra
is not a pharmacological substance, and it has no direct therapeutic effect on
the diseases that cause chronic ulcers. Nevertheless, Integra has a
systems level ability to control the local expression and effects of
disease. Like most complex physiologies, injury, disease, inflammation,
ulceration, and all of their attendant therapies constitute a complex
non-linear dynamical multi-control system. Normal tissue, active stable
disease, rampant disease, normal wound healing, retarded wound healing, and
active ulceration are all basins of attraction or local stability on the
chaotic attractor of this system’s state space. In chronic pathological
wounds, sick controllers with impaired degrees of freedom cannot easily
regulate a desired healthy state. Perturbations of the system by disease,
trauma, or treatment can have variable, unpredictable, and even contrary
effects. The body has many systems for fighting, controlling, and
eliminating disease, but in the chaotic pathological wound, these systems
cannot prevail. Integra does not directly cure any diseases, but its
ability to control a wound (inflammation, thrombosis, exposure, bioburden,
etc.) is profound enough to permit normal physiological systems to recover,
regain the upper hand, and overcome residual pathology. Via complex
non-linear interactions, Integra controls soft tissue pathology and ulceration
and keeps them controlled. (Cases 2, 3,
7, 8, 9, 11, 18, 20).
Resistance to recurrent
disease.
Continued management of underlying
diseases and risks is mandatory for any wound in any patient, before, during,
and after the acute phases of healing and reconstruction. This serves
both the general health of the patient and the continued health of the closed
wound. However, in spite of good care, some patients will have unexpected
injury or illness or flare-ups of disease.
Many will lapse in their follow-up and preventive care. Like
anything else, Integra can be damaged by trauma such as lacerations or
pressure. Integra can also be affected if disease and ischemia are severe
enough. However, relative resistance to
new disease has been a repeated observation when treating patients with
inflammatory and lytic disorders such as venous or rheumatoid ulceration. This resilience may be due to two factors: it
might not be the Integra, but rather absence of underlying adipose which is
where ulcerative panniculitis typically occurs; or it might be the Integra,
because as a more embryonic type of tissue, it might have some inherent
resistance to these diseases. (Cases 2,
7).
Integra
General Indications
Integra has numerous indications
and modes of use. (1) As an effective artificial
skin, Integra can provide critical coverage for salvage
of life and limb. Placed on a wound, it
blocks recognition of injury, quenches inflammation, controls pain, pathergy,
edema, and fluid fluxes, stabilizes general metabolism with large injuries
40, 41, significantly reduces nursing requirements, and accelerates
recovery and rehabilitation 26, 27, 42 - 44. This is of value for any large wound, and
Integra is often life saving for large burns, deglovings, and fasciitis
20, 26, 27, 34, 35, 42 - 50. This
property also permits elective resection of large areas of skin for non-acute
conditions such as lymphedema 51.
(2) As a skin regenerant, Integra is valuable for any soft
tissue reconstruction where quality of the skin and avoidance of
scars and contractures is desirable. (3
) Integra also provides essential coverage, closing exposed
visceral and skeletal structures. The
conventional arts of wound closure require that these be covered with
vascularized wound healing competent tissues in the form of flaps or other
complex repair. As an artificial skin, Integra
provides interim protection for these structures, and as an agent of
regeneration, it creates the final coverage, supplanting many flaps. (4)
When closing pathological wounds, Integra also acts in both modes
(artificial skin and skin regenerant), the concept of a biological
superdressing. In these
situations, the artificial skin actually has a true therapeutic role, allowing
residual inflammation and pathology to subside, controlling the risks which
threaten wound healing and surgical repair.
Many chronic ulcers also have exposed essential structures, as evident
in the patients in this study, a consequence of disease and of the thorough
wound excision needed prior to placing Integra.
Worldwide, clinicians and investigators have reported favorable
experiences with Integra when used in any of these modes 20 - 56,
most of it with acute trauma and elective reconstruction in healthy people, but
also in chronic wounds 50,56.
Rationale and
Indications for Chronic and Pathological Wounds
Chronic and pathological wounds
are prolonged and poorly healing because there is underlying disease which
either perpetuates the ulcer or inhibits healing. While control of underlying disease is
mandatory in principle, in practice it can be impossible to completely
eliminate adverse factors such as autoimmune inflammation, arterial
insufficiency, and the effects of radiation.
The disease itself may cause anatomical complications or caveats, such
as rheumatoid synovitis causing exposed ankle tendons or severe atherosclerosis
eliminating the usual flaps used to cover open skeletal structures. It is these wounds which are subject to
pathergy and wound healing failure, leading to prolonged care, multiple failed
procedures, and prolonged frustration and expense. For unwary surgeons, autogenous materials are
prone to be wasted, and donor site complications may enlarge the original
wound. Integra’s properties allow it to
manage or solve these problems. Often
superior to conventional methods of wound closure (repair, grafts, flaps), and sometimes
the only option, Integra should be used preferentially for the following
circumstances. (Case studies which
illustrate these points are referenced).
High risk history and
susceptible disorders. Use Integra: if the patient has a history of failed surgery
for the ulcer or related condition; if
the patient has a history of failed surgery for other reasons; if there were wound and soft tissue
complications of prior surgery or trauma (pathergy); if prior reconstruction or wound closure has
become re-ulcerated; if the patient has
arterial disease, immunopathic diseases, longstanding or advanced venous
disease, or hypercoagulable and micro-occlusive disorders, especially if
severe, difficult to manage, or actively flared up. (Cases 1, 2, 3, 8, 10, 15, 18, 20, 21, 23,
24).
High risk ulcer profile. Use Integra: if the current ulcer has failed or progressed
in spite of reasonable prior care; if
examination shows persistent inflammation, necrosis, lysis, and progressive
ulceration; if there was pathergy and
progressive necrosis after debridement and supervised care; if pain is difficult to control; if skeletal or visceral structures are
exposed or can be anticipated after debridement; if periwound transcutaneous oxygen levels are
seriously diminished; if the ulcer or
its location are ordinarily considered to be at high risk for amputation. (Cases 2, 7, 8, 11, 13, 15, 16, 18).
Inflammation and disease
persist. If the physician has done everything that
current art and science permit to treat the disease and control the wound, but
inflammation or active ulceration persist, then conventional repair or grafts
with living materials are apt to fail, and Integra should be used. Integra is not only preferred because it is
not alive, but it is also therapeutic, and rapid subsidence of pain,
inflammation, drainage, and ulceration can be expected. (Cases 1, 2, 3, 7, 8, 9, 12, 13, 14, 15).
Topical care not succeeding. It is necessary
with any chronic wound to have a preliminary treatment phase in which diagnosis
is made, risk factors are corrected, and basic wound care is initiated. By the time these activities are concluded, a
few weeks of observation will have revealed if the wound is wound-healing
competent and beginning to proliferate an active wound module. If the wound is incompetent to heal under
ordinary circumstances, Integra ought to be used in lieu of conventional
surgery. (Cases 2, 3, 4, 5, 6, 7, 8, 9,
11, 12, 13, 21).
Control of symptoms. If severe pain is
present due to persistent pathological inflammation (i.e. the inappropriate
inflammation of immunopathic or similar disorders, as opposed to the reactive
inflammation of controllable factors such as trauma and bioburden), wound
excision and closure with Integra will cure the pain, and it should be done for
humane symptomatic relief. (Cases 2,
11).
Surgical complications or
wound failure anticipated. Use Integra: if residual pathology, inflammation, or
ischemia threaten complications of conventional flaps, grafts, and
repairs; if prior attempts to do surgery
for the same condition failed; if the
established diagnoses or confirmed ischemia carry a high risk of pathergy and
surgical complications. (Cases 1, 2, 3,
7, 8, 10, 11, 15, 16, 18, 19, 20, 21, 22, 23, 24).
Skin grafts ineligible. Skin grafts are
technically convenient but biologically complex, being completely dependent on
a healthy and wound healing competent host wound. Conditions such as ischemia, devascularized
structures, and minor residual inflammation nullify the use of skin grafts
because the grafts will die or not adhere.
Not being alive, Integra does well in these circumstances. (Cases 1, 2, 7, 8, 10, 11, 15, 16, 18, 19).
Flaps ineligible or at high
risk.
As normal vascularized tissues which are independently capable of and
responsible for healing a wound, flaps usually solve what grafts cannot. However, flaps are not always safe or even
technically possible. Integra can be
safe and successful when flaps cannot or should not be used: if local anatomy has a limited choice of
flaps (such as the distal leg and ankle);
if eligible flaps are in the zone of risk and therefore likely to
fail; if active inflammatory diseases
threaten wound complications along new incisions; if vascular disease and ischemia threaten
flap necrosis; if atherosclerosis or
hypercoagulability or general patient condition make a free flap unwise. (Cases 3, 10, 11, 15, 16, 18, 19, 23, 24).
Exposed essential
structures. One of the preeminent indications for flaps
is the closure of visceral, skeletal, and alloplastic structures. If flaps are ineligible for any of the above
reasons, Integra is a highly dependable substitute, because of its role as a
high quality artificial skin, and because of tangential histoconduction and its
ability to bridge a non-living hiatus. There
is no limit to the extent of coverage if Integra is placed on living
structures, such as abdominal organs or healthy bone. It is unknown how much of a void it can
bridge, but case 16 describes the details of closing 2-3 cm of exposed metal
hardware. (Cases 1, 3, 4, 6, 8, 9, 10,
14, 15, 16, 19, 21, 22, 24).
Biological coverage
desirable. Biological dressings are used for interim
wound closure, to control persistent mild inflammation or to provide interim
protection of a wound in preparation for later closure. If the needs for wound closure are truly
transient, if the wound is going to be subject to further debridement, or if it
is going to disappear after later repair or flap inset, then less costly
materials such as cadaver allograft or Biobrane are better suited. However, if the involved wound surface will
need its own closure, then Integra is preferable. It will eliminate intermediate steps, because
it serves both purposes, short term biological coverage and definitive
reconstruction or wound closure. (Cases
11, 12, 16, 17, 23)
High risk patient. Integra placement
is quick and safe, the only “cut and bleed” risk to the patient being the wound
excision. Subsequent skin grafts have no
more risk than the graft donor sites.
Integra should be used in lieu of conventional repairs for any patient
who is sick or a poor anesthesia risk.
If disabilities and psychosocial circumstances warrant minimum
surgery-induced disruption of daily affairs, Integra can be the simplest yet
most dependable approach to wound closure.
Integra is the ultimate “play it safe” wound closure option. (Cases 1, 2, 21, 22, 23).
Large surface area. Large wounds need
correspondingly large flaps or grafts.
These create potential post-operative problems of increased
inflammation, physiological stress, pain, drainage, soiled dressings, septic
risk, nursing requirements, and functional inhibitions. In a sick or
disabled person, simultaneous large donor sites for large wounds can be “too
much”. By using Integra, net physiological load on the body is significantly
reduced. The primary defect immediately ceases to be a wound
(physiologically speaking), donor sites are eliminated, and the later skin
grafts done by themselves are easier to manage. Integra should be
considered whenever it is desirable to limit collateral injury, minimize wound
surfaces, minimize acute physiological stress, and simplify post-operative
symptoms and nursing. (Cases 5, 11, 12).
High risk donor sites. Flap and graft
donor sites can be at risk because of disease, location, ischemia, inflammation,
and any other factor discussed above.
Even if a donor site can be harvested without wound complications, there
can be functional contraindications. For
example, the latissimus dorsi muscle is crucial for competent use of crutches,
walkers, and wheelchairs, all necessary orthotics for many chronic wound
patients. Yet a latissimus free flap is
one of the first choices for many plastic surgeons wanting to cover a complex
lower extremity wound. Preservation of
health, function, and lifestyle are the real goals, not simply wound closure
for its own sake. If it is desirable to
avoid donor sites, Integra eliminates this risk while giving equivalent or
superior coverage of the target wound.
(Cases 2, 10, 15, 18, 22, 23, 24).
High risk for recurrent disease. Patients with
chronic wounds have chronic disorders, and recurrent ulceration is a risk,
especially for immunopathic, venous, lymphatic, and hematological
disorders. Because Integra reconstructed
areas seem to be somewhat resistant to recurrent disease, it is superior to
scars and conventional skin grafts which are prone to re-ulceration. Integra should be used in areas of unstable
skin and scar with a history of either repetitive ulceration or risk for future
ulceration. If a wound is still small
but is surrounded by wide areas of extensive trophic changes, dermatitis, and
liposclerosis, areas at risk for broader ulceration, then strong consideration
should be given to pre-emptive dermatofasciectomy of the entire area and skin
reconstruction with Integra (usually involving the distal leg and ankle). (Cases 1, 2, 5, 7, 8, 12, 13, 14).
Avoiding scar and improving
reconstruction. Regardless of the reason for Integra, it
controls scar, and late revisions for scar contracture, such as after burns,
are infrequently required. For chronic
wounds, if the area involves joints, hands, face, neck, genitalia, or any other
mechanically compliant area, then Integra should be given primary consideration
unless some suitable flap or direct repair can do an equivalent or quicker
job. (Cases 10, 17, 20).
Simplifying care and
preserving function. Upon placement of Integra, the wound is
immediately closed. Pain, drainage, and
other symptoms cease. Nursing
requirements are nil, permitting almost all care to be outpatient, requiring
only periodic (weekly) dressing changes.
For patients with obligations at work or home, who must preserve
function and lifestyle, who must travel, who live remotely and cannot come for
frequent medical visits, or in whom other illnesses take precedence, Integra
can be used because of its ability to simplify care. (Cases 5, 12, 14, 16, 17, 23).
Absent risk factors, a superior reconstruction. Integra can heal
problematic wounds. It can succeed where
flaps would ordinarily be used, sometimes with superior results. It can succeed where flaps cannot be used. It can avoid sequelae of conventional surgery
(scars and contractures after skin grafts, or revisions and “debulking” of
flaps). It can do so with neither risks
nor donor sites nor inpatient care.
These are valuable properties regardless of the circumstances of the
primary wound. For benign
non-pathological wounds, situations where the ordinary means of repair would be
safe, reliable, successful, and customary, Integra might nevertheless be
preferred, precisely because of its own advantageous properties. In some of the case studies (1, 10, 12, 15,
16, 17, 19, 20, 21, 22, 23, 24), imagine that the given situations occurred due
to trauma or surgery in young healthy people, and then contemplate whether
customary grafts and flaps would have been easier, safer, less resource
intensive, or would have given better results that those that were actually
obtained with Integra.
Use of
Integra, Discussion by Diagnosis and Pathology
Macro-arterial Arterial
insufficiency, usually due to atherosclerosis, was the most common risk factor
in this group of patients. It was also
the group with the highest percentage failure and lowest percentage group 1
success (along with the diabetic group, all of whom had arterial insufficiency
as one of the secondary diagnoses, Table 3b).
Atherosclerotic macroarteriopathy is distinctive in that it is an
anatomical condition of blood vessels but not an active disease which causes
ulceration nor damages intrinsic wound repair physiology. The relative degree of arterial insufficiency
governs how easy or difficult wound repair and surgery will be. Thus, the results with Integra fell across a
spectrum of outcomes from total success to total failure. Note that length of treatment is one of the
lowest (Table 4), indicating that in the absence of an active ulcerative
disease, these patients either got better quickly or they failed altogether,
contingent on the severity of the insufficiency. Ideally, arterial insufficiency should be
corrected prior to any wound closure surgery, Integra or otherwise. To the extent that revascularization restores
wound healing competence, an ulcer might be closed by topical care only or by customary
one-stage grafts and flaps. Integra is
valuable for two reasons in patients with more severe disease. The first is its ability to control wound
conditions, averting the pathergy that ordinarily complicates this
condition. As a non-living material it
will not fail the way that grafts and flaps might, and it is ideally suited to
the retarded or delayed histogenesis that can accompany arterial
insufficiency. The second reason is its
ability to bridge exposed essential structures, performing well in lieu of
conventional flaps which might be unavailable or too risky.
Efficient selection and management
of these patients would be facilitated by knowing when arterial insufficiency is
so severe as to ensure failure. There
was insufficient data to permit precise correlation of outcomes with clinical
measures of arterial circulation, but some general guidelines can be
stated. For ankle-brachial indices
(ABI’s) of 0.7 to 0.8, conventional repair, grafts, and flaps are permissible,
although Integra might be preferable for the closure of tendons, bones, and
joints in lieu of higher risk flaps.
ABI’s below 0.2 to 0.3 are likely to result in failure regardless of
method. In these circumstances, if
operative closure of any kind is attempted, Integra is not only safe and of
marginal risk, it is also probably superior to conventional repair in the
likelihood of success, losing nothing but time if it is attempted. Nevertheless, at these low ABI’s, failure is
mostly assured. It is in the middle zone
where conventional surgery is likely to fail or carry substantial risk that
Integra is likely to be successful. In
just over half of these patients, hyperbaric oxygen therapy was used to restore
missing oxygen, typically started at the time of Integra placement or up to a
few weeks in advance, with courses of treatment typically 20 to 40 sessions
depending on progress. Adjunct
hyperbaric therapy was felt to be worthwhile, although many patients not having
this therapy also did well. Salvaging
complicated stumps, such as coaxing a below knee amputation wound to heal
rather than conversion to an above knee amputation, is a particularly valuable
capability (4 patients in this group had successful salvage of an amputation
already performed by another surgeon for which higher amputation had been
recommended, and 5 patients avoided immediately impending amputations scheduled
by other surgeons). (Cases 10, 18, 19,
21, 22, 23, 24).
Hypercoagulable and other
micro-occlusive. These are a wide range of metabolic and
hematological disorders which cause micro-occlusion, but no other active
injurious pathology. They include the
prethrombotic and immune mediated hypercoagulable states, hemoglobinopathies
and disorders of formed blood elements, dysproteinemias, and metabolic
disorders such as calciphylaxis. Pathophysiological
issues and discussion are comparable to atherosclerotic ulcers and necrosis,
including severe ischemic pain, pathergy, refractoriness and multiple treatment
failures, and threatened or prior amputations.
But there is an important difference:
these conditions are easier to manage.
They tend to occur in younger otherwise healthier patients, and the
problems or their effects are correctable, such as treating hypercoagulable
patients with anticoagulants. The
consequence is that patients in this category had a high success and a low
failure rate. Because these are ischemic
conditions, hyperbaric oxygen is of potential benefit for short periods
(therapeutic anticoagulation, natural thrombolysis, vascular recanalization,
and regenerative angiogenesis mean that ischemia corrects itself in these
conditions, so hyperbaric therapy need not be done for more than a week or two,
as was done in 50% of these patients).
(Cases 2, 3, 8, 11, 20).
Diabetes. Diabetes does not
cause any type of inflammatory or metabolic injury which directly inhibits
wound repair. Thus, for the 19 patients
having diabetes as a secondary diagnosis, it was simply coincidental and did
not influence good or bad outcomes. For
the 5 patients having a characteristic “diabetic ulcer”, results were mostly
poor. Arterial insufficiency was a
contributing factor, but in those patients with adequate circulation, Integra
was technically successful, regenerating and accepting skin grafts. For those 5 ulcers on weight bearing plantar
surfaces, the real problems were mechanical load and patient compliance. Even though the material performed properly,
these patients were categorized as failures (table 3b) because the Integra did
not contribute to a good final outcome.
The foot itself is not a problem.
Integra is extremely dependable on non-weight bearing foot surfaces, and
in those patients who are responsible about activities and orthotics. Lessons learned were (1) if a patient cannot
be compliant during a plantar reconstruction, it might be best not to begin at
all (true for any type of diabetic foot reconstruction), and (2) even when
Integra heals properly under the calcaneus or other plantar weight bearing
surfaces, it will ulcerate when subjected to ambulation and weight bearing. Comorbidities such as arterial insufficiency
and neurological or psychological inadequacies amplify these risks, so an
Integra reconstruction on the weight bearing plantar surface is probably best
avoided in diabetic foot patients.
(Cases 18, 19, 21, 23, 24).
Venous and lymphedema. These disorders
are grouped together because (1) they share some common pathophysiological
mechanisms, (2) the chronic trophic tissue changes and other anatomical
pathology are similar, and (3) treatment principles and frustrations are
similar. Many patients with these
disorders have never had systematic sustained care, and most will improve
without surgery if proper care is initiated.
For those who have had comprehensive good care and then need surgery,
skin grafts often succeed (along with continued compression and maintenance
care). In the 18 study patients who had
these as primary diagnoses, the indications for Integra were based on advanced,
refractory, complicated disease: (1)
long duration with prior failed skin grafts,
(2) advanced liposclerosis and scarification of the sural fascias which,
after excision, left bare muscle, tendon, and synovium upon which to place
grafts, (3) pre-excisional ulceration
into skeletal structures such as tibialis and peroneus tendons, the bony
malleoli, or the ankle joint. Good
preparation is essential, including vigorous wound and skin care, topical or
systemic steroids (control of venous vasculitis and dermatitis) and strict
compression (control of edema and venous hypertension). If venous interruption was not previously
done as an independent form of treatment, it should be done (as anatomically
required) at the time of wound excision and Integra. In the compliant patient, these goals are
easily achieved, and the success rate was one of the highest. (Cases 4, 5, 6, 14).
Immunopathic. Immunopathies are
active diseases. Their designations as
connective tissue and collagen-vascular disorders underscore the effects they
have on these tissues, and progressive lytic ulceration of skin and fascias and
wound healing impairments are common in the leg, ankle, and elsewhere. Panniculitis and ulceration are sometimes the
only overt manifestation of disease.
Immunopathic ulcers are often misdiagnosed and mistreated, but even when
diagnosis and care are properly instituted, resolution of these wounds can be
difficult and prolonged (Table 6). While
it is true that rheumatoid and similar patients have prosthetic arthroplasties
and other elective surgery performed successfully, the presence of chronic
ulceration indicates locally advanced pathology and active systemic disease
which consistently stymie attempts to treat.
Histories of many years or decades duration and failed prior wound
surgery are common. The prospects for
successful surgery using nearby autogenous tissues are very small. Many of the patients in this study, being
older, had concurrent venous or arterial disease of varying degrees. Because the pathology of these disorders
causes lytic ulceration, it is common for adipose fascias to be dissolved,
leaving muscles, tendons, retinacular
ligaments, bones, and joints uncovered.
Therefore, most of the study patients with these diagnoses had exposed
structures compounding the issue of active refractory pathology. Seven of the patients had extensive or
circumferential leg ulceration comparable to the one in case study 12. Effective management begins with control of
the underlying disease to the extent that it is possible. It is not always possible though, and
residual dermatitis, panniculitis, positive serologies, systemic symptoms, and
wound inflammation and active ulceration may prevail. It is in these circumstances, where
aggressive treatment has improved but not eliminated pathological inflammation,
that the concept of a biological superdressing is particularly applicable. Integra’s ability to subside the residual
inflammation, then reconstruct skin and cover exposed essential structures had
consistently good results in these patients.
When some of these patients had subsequent flare-ups with new wounds,
the Integra reconstructed skin was spared from re-ulceration. (Cases 1, 2, 7, 12, 15).
|
Table 6: Duration
of care of immunopathic ulcers
|
This
data is taken from one of the hospital based wound clinics where many of the
study patients were cared for. This
shows length of care for all clinic patients, collated for administrative
purposes, compiled for a two year period roughly coinciding with the last two
years of the current study. These data
confirm that immunopathic patients are difficult to heal, lengths of
treatment exceeding other categories by substantial factors. |
Mechanical, anatomical,
trauma, and surgery. Typical histories in this category included
pseudarthrosis, ulcerated contractures, and open shearing tendons. These patients tended to be younger, and
ulcerative or inflammatory diseases were not a concern. Essential coverage situations were not only
common but they were often part of the actual wound pathology, since it was
exposure of these structures which were inhibiting wound repair or
closure. In these patients, Integra was
opted because of limited options for flaps, risky flaps due to concomitant
vascular disease, and anatomic locale such as the dorsum of the hand or across
joints where a thin but compliant reconstruction was desirable. (Cases 9, 15, 16, 19, 20).
Granulomatous, infectious,
and miscellaneous. There are some incidental chronic ulcers due
to infections, metabolic disorders, and other infrequent pathological
conditions. These include atypical
infections (mycobacteria, fungi, actinomycetes), granulomatous inflammation,
osteomyelitis, and localized calcium dystrophies, with or without other
risk. These are all comparable to
mechanical and traumatic ulcers in that disease is localized, that it can be
eliminated by adequate excision and adjuvant therapy, and that general health
and wound healing competence are not affected.
In all of these situations, excision of disease, good wound preparation,
proper adjuvant treatments, and control of motion allow Integra to be used to
cover essential structures and to induce stable healing wounds.
Radiation and malignancy. In the few
radiation ulcers in this study, time to skin grafts and time to completion were
long, but Integra nevertheless healed.
Because radiation damages the proliferative potential of local wound
module progenitor cells, normal wound healing can fail. Integra has the virtue that, even if it is
healing slowly, it provides superb protection to the wound, allowing
regeneration to occur at whatever slow rate it will. To the extent that the pioneers and some
angioblasts are migratory cells ultimately derived from bone marrow, Integra
might theoretically succeed even over highly irradiated tissues (> 6000
cGy). One of Integra’s properties, fulfilling
the concept of “tissue engineering”, is that the matrix can be a carrier of
seeded cells, allowing the surgeon to supply what the host wound cannot. This concept was used successfully in two
patients with bare irradiated calvarium (case 25, figure 30). Integra was also used in two patients in this
study for wound closure and essential coverage after wide resection of
ulcerated tumors, but it was never used to close gross tumor itself. Its use in that regard can be considered the
same as closure of any surgical or anatomical defect.
|
Figure
30 a (top left), b (top middle), c
(top right), d (bottom left), e (bottom middle), f (bottom right) (a) This 82 year old
woman had scalp skin cancer treated by radiation, 6500 cGy. The resulting chronic ulcer of parietal
cranium was vascular and viable but not wound healing competent. The situation was compounded by advanced
Paget’s disease of bone, causing deformity, atrophy, and immobility of the
surrounding scalp making local flaps impossible. Various large
remote flaps would have succeeded, but the patient was only willing to
consent to minor outpatient and clinic procedures. The solution was to
use Integra as a carrier of healthy cells. The reconstruction started
by placing a small fenestrated plastic chamber under abdominal skin.
Two weeks later, proliferative tissue within the chamber has removed, then
mashed, partly trypsinized, and filtered. The resulting paste of
mitosis-competent mesenchymal cells was worked into a piece of Integra which
was then applied to the debrided scalp wound.
This image is the scalp ulcer after debridement, just prior to placing
the seeded Integra. (b) This is the matrix
at 2 weeks. Some small unmacerated yellow fat lobules are present from
the original seeding. Scattered through the matrix, especially center,
right, and top, is a multifocal bloom of opacifying loci distinct from normal
Integra regeneration patterns. The
matrix may have been populated in part by wound-derived cells, either blood
borne or locally resident, but this image suggests that it was mainly the
cell transplants which generated the neodermis. (c) When the first
piece of Integra was regenerated, it was opted to place a second piece to
build a thicker lamina of new tissue.
This image is at 4 weeks, at the time of the exchange. (d) This is the second
piece of Integra, completely regenerated and ready for skin grafts. (e) The skin grafts
appear normal here, 2 weeks after their placement. (f) The skin grafts
healed transiently. Seen here 5 months
later, dystrophic pagetoid bone is starting to extrude through the
reconstruction, beginning to cause new ulceration. The patient has
since adopted a program of chronic maintenance care for the open areas, and
she remains healthy. While this was an
odd and unlikely set of circumstances, it demonstrates that Integra can be
used as an in situ reactor or incubator to engineer a new tissue using
cultivated cells not derived from the substrate wound. |
Adjunct. Patients in this
category had large or long-standing defects needing a complex reconstruction. Integra was not the preferred option to
directly close the primary wounds, and large or delayed flaps were used for
that purpose. However, Integra was an
ideal companion to the flaps. They were
used in coordination for these reasons:
(1) A flap donor site might have its own needs for closure. (2)
For flaps in intermediate stages of transfer, Integra can close and protect an
unsatisfied open end, and it can cover a bare undersurface to simplify care and
prevent contraction. (3) Integra can close the donor wound under a
delayed flap, preventing revascularization where delay incisions have already
been made, and simplifying the final transfer.
(4) A mosaic reconstruction can be done, using a small, safe, dependable
flap where it is most crucially needed, and using Integra to close remaining
areas where the flap cannot cover. If
Integra is used for these purposes, in lieu of skin grafts, it will protect an
open or delayed flap, improve the safety and dependability of the flaps, make
the flap donor site easier to manage, avoid extra donor sites, simplify a
complex reconstruction, improve quality of the final result, simplify
post-operative care, and minimize wound area, pain, drainage, and nursing needs
(this was especially useful for the 2 children in this series, case 17).
Use of
Integra, Discussion by Anatomy
Head, trunk, upper extremity. As would be
expected, wounds and reconstruction on upper parts of the body tended to do
well. The indications for Integra were
comparable to those already discussed:
refractory ulcers by history, failed prior procedures, exposed essential
structures, lack of suitable local flaps, and the desire to limit donor
sites. Integra was particularly
effective for closing the dorsum of the hand.
Situations which by convention require groin, abdominal, radial forearm,
and similar flaps also require staged transfers, imposed disabilities, risk to
the forearm and hand, difficult nursing and daily activities, and staged
reductions of fat once the flaps are healed.
Integra results in a thin and compliant tissue comparable to normal
dorsal hand skin, with neither donor sites, nursing and functional problems,
risk of flap necrosis and dehiscence, nor late revisions. Whether for trauma, chronic wounds, or
elective reconstruction, Integra should be considered the option of choice for
restoring skin on the dorsum of the hand and wrist.
Lower extremity. Most chronic
wounds are on the lower extremity, reflected in the distribution of these
cases. Special circumstances and caveats
apply when doing any reconstruction on the lower extremity, Integra or
otherwise. Integra tends to mitigate
pathology and inflammation, which is why it is effective when other treatments
have failed, but the following issues must always be considered. Never overlook the possibility of concurrent
arterial or venous disease, or any other combination of multiple risks, and
treat each risk accordingly.
Regeneration times may be prolonged, 6 - 7 weeks sometimes. Edema control and graft fixation are
essential. Use splints or boots to
control motion of joints or major tendons that are covered with Integra. Avoid pressure injury due to tight bandaging
around the foot and ankle. Do whatever
is required to protect the reconstruction, but do allow ambulation and preserve
function as long as mechanical loads and strains on the graft are completely
eliminated in responsible patients.
Concurrent treatment of the underlying disorder must continue (e.g.
steroids for immunopathic disorders, anticoagulants for hypercoagulable
disorders) depending on the status of the disease and complications of
treatment.
Exposed structures. Conventional
plastic surgery principles dictate that open bones, joints and bursas, tendons,
viscera, and alloplastic materials all be covered with flaps, i.e. vascularized
composite tissues capable of independently healing the recipient wound. Skin grafts and other living or autogenous
materials can be, at best, only a temporary biological dressing. As a skin substitute, Integra provides
superior acute coverage of these structures.
Because of its ability to conduct histogenesis tangentially, it readily
bridges these defects. In this study,
Integra closed 90% of all such instances (table 3d). It did so when flaps were not possible,
without donor sites and donor morbidity, and without late revision. Since exposure of these structures is what
prompts many surgeons to suggest amputation, Integra resulted, in principle, in
many saved limbs. Notable points are the
following.
Integra does well on bone, because
healthy bone is healthy tissue, mechanically stable and alive, capable of
sourcing cells and circulation into the graft (cases 7, 8, 14, 15, 18, 19, 21,
22, 23, 24, 25). If Integra over bone
(or any tissue) turns black, it means that the subjacent bone is dead. Further tangential bone debridement and
reapplication of Integra will succeed.
Integra performed well over open joints, especially small joints of the
hands and feet. Until healed, control of
motion by bandages or orthotics is essential.
Integra performed well over tendons (cases 1, 2, 3, 5, 7, 10, 12, 16,
20, 23). It does especially well over
small extensor tendons. Tendon diameter
does not seem to be necessarily important, but a combination of size and
excursion is, with peroneus tendons just above the malleolus and tibialis
tendons across the ankle being most likely to require secondary coverage by
flaps or new Integra. Note that if
Integra can control and heal a large wound to the point that only a small
accessory flap is needed to close a small peroneus exposure (category 2
incomplete healing), this is a clinical success (case 4). Integra does uniformly well over the achilles
tendon, as long as the tendon is properly debrided. Pressure ulcers of achilles tendon and of
heel (calcaneus) are common, independently or accompanying each other, and when
surgery is indicated, Integra readily solves both problems (cases 1, 21, 23,
24). Visceral organs and alloplastic
hardware are best closed by flaps, but when flaps are unavailable or patient
risk contradicts their use, Integra does a remarkably good job of closing and
restoring skin over them (case 16). One
of Integra’s values is that it buys time for the surgeon and patient. It can protect a wound or structure while ancillary
matters are stabilized or while a final flap is being delayed. If, while being used as an interim skin
substitute, it regenerates and heals the wound, then the parallel plan of
closure can be abdicated.
Technique and Management
Good
outcomes with Integra are contingent on technique and details of
management. The nominal methods of use, which
are comparable to ordinary skin grafts, are described in prior literature and
in the package insert. This section
discusses additional details especially relevant to its use in chronic wounds.
Control disease and prepare the
wound. All chronic wound patients must have
accurate diagnosis and treatment of underlying disease and risks. There must be thorough pre-operative control
of inflammation, ulceration, debris and bioburden, and edema (as best as the
disease and available treatments permit).
Integra can control some residual pathological inflammation, but to
ignore proper wound preparation invites abscess and loss of the material. The most common preparatory treatment profile
for patients in this group was twice daily hygiene and silver sulfadiazine
dressings, edema control by elastic or multilayer bandaging, and incidental
therapies related to individual diagnoses.
Excise the wound. Regardless of how
well the wound has been prepared and how healthy it looks, Integra must not be
placed on an existing wound surface. Not
only does this risk infection, but if Integra is placed on a proliferative
wound module of cells already committed to conventional inflammatory fibrous
repair, the full late phase benefits of a compliant scarless tissue will not be
realized. At the time of surgery, the
entire existing wound must be completely excised. If anatomical circumstances preclude safe
excision (e.g. the wound is on open internal organs), then thorough curettage
should be done to remove all “granulation tissue”. Integra is a surgical implant, not a wound
dressing, and it must be accorded due respect.
Forms and availability. The original
product, packaged in isopropyl alcohol, is available in three rectangular
sizes, 4x5, 4x10, and 8x10 inches. As much
as is needed can be opened and applied to cover the prepared wound after first
rinsing out the alcohol. A new package
using only electrolyte buffer is recently available. If needed, the Integra sponge can be gently
scraped from the silicone and used by itself for extra thickness or bulk
filling in small bursas or cavities.
Antibiotics. Antibiotics are
used by many surgeons, usually as part of the preliminary rinse. All patients in this study had concentrated
antibiotics impregnated into the sponge after the rinses were complete (Table
7), supplemented by several days of systemic antibiotics (oral for outpatients,
intravenous for inpatients). The low,
nearly zero infection rate is attributed predominantly to good pre-operative
preparation, complete excision of the wound, and good fixation and
compression. Whether antibiotics are
useful or not is a matter of faith, but they are a cheap and safe hedge against
an undesirable complication.
|
Table 7: Antibiotic
use and concentration
|
Antibiotics
can be impregnated into the Integra matrix.
Choice of drugs is arbitrary.
The investigator’s practice has been to use 1 gm vancomycin dissolved
in 3 vials of gentamicin solution (6 cc’s, 240 mg), one such mix applied to
each Integra sheet (4 x 10 or 8 x 10 inches) after rinsing and
preparation. This mixture was chosen
based on its antimicrobial spectrum and the simple reality that these are the
drugs stocked in the operating rooms where the author practices. This practice has been safe, as measured by
serum drug levels. Representative
patient A had closure of the leg for extensive rheumatoid ulceration (750 sq
cm Integra), and patients B and C had dermatofasciectomy of the leg for
primary lymphedema (2000 sq cm Integra).
The total “dose” is indicated.
Serum drug levels were measured at the indicated times after
surgery. Serum drug levels remained
low, at or below normal therapeutic trough levels. There have been no instances of oto- nor
nephrotoxicity, but the practice ought to be amended in the face of renal
insufficiency (or alternate drugs used).
It seems that the Integra is either binding or sequestering the drugs,
presumably maintaining high local concentrations without significant systemic
exposure. This discussion does not
advocate a specific formula or choice of drugs, but simply demonstrates that
the practice is safe. |
Application to wound. The Integra must
conform to and contact the wound surface.
Tension within the material will shear the sponge from the silicone, so
the material must not be stretched. The
material as is is sufficiently deformable to let it conform to most wound
surfaces, but it can be folded, pleated, darted, and mosaicized in any way
desired so that unstrained material is everywhere in contact with the
wound. It can be affixed with sutures,
staples, or any suitable alternative.
While not used on any of these patients, others have reported good
success using fibrin glues to cement the product on the wound.
Fixation and compression. Fixation and
compression are of paramount importance.
The principles and art are no different than for affixing any skin
graft. Elastic bandages, padded
“tie-over” dressings, and vacuum devices, interphalangeal pins and other
hardware fixation, and splints, boots, and other orthotics are all used,
depending on circumstances, to ensure that the material adheres to the wound
without shear, and that hematomas and seromas do not accumulate. In the patients in this study, elastic
compression bandages, occasional tie-over dressings, and plaster or
prefabricated splints across joints were the common forms of fixation.
Interim management &
observation. If disease has been controlled, the wound
properly prepared and excised, and the graft properly fixated, then
post-operative care and concerns are minimum.
Because histogenesis is observable through the silicone, it is necessary
to periodically examine the graft. For
these patients, examinations were done at one week intervals during normal
clinic hours, consisting of unwrapping and then rewrapping new compression
bandages. If there are no problems with
the graft or the dressings, examination intervals of 2-3 weeks suffice. When the graft is fully opacified with new
tissue, skin grafts are ready to be placed.
Time until skin grafts is usually 3 - 4 weeks in young healthy people
with upper body reconstructions. For chronic
wound patients in this study, intervals were mostly 5 – 6 weeks (table 4). Activities and lifestyle are permitted to the
extent that splinting and edema control can be maintained.
Separated silicone. As regeneration nears completion, new tissue dislodges the
silicone overlayer. The nominal usage of Integra is to place skin grafts
when histogenesis is complete, but before the silicone separates.
Silicone sometimes separates before grafts can be placed. Blistering of
the silicone is irrelevant, but if it opens onto an edge, then some minor
inflammation and benign sub-silicone abscess can result. Removing or
windowing the silicone and beginning regular hygienic topical care will keep
the matrix healthy, regenerating, and ready for the skin grafts. Usually
of no consequence, this rarely affects whether the wound heals, but it can risk
getting some inflammatory wound module and scar. Another problem is premature
silicone ejection due to benign foreign body giant cell reaction. This should not be confused with acute
inflammation or infection, and it will not jeopardize the skin grafts (figure
28-5b).
Overgrafts. When
dermatogenesis is complete, the silicone is lifted, and thin epidermal
autografts are placed on the neodermis.
These skin grafts are managed as any other, but thin grafts (3 – 8
thousandths of an inch) are typically used, trying to minimize the amount of
mature dermis which is transplanted, and minimizing donor site problems in
these at-risk patients. Customary graft care is practiced, and small
remaining bare areas will gradually epithelialize. Grafts can heal within 2 – 8 weeks in healthy
patients, but in more problematic wounds, complete epidermal healing times can
be several months (table 4a). If Integra is open, either because of
silicone separation or failed skin grafts, then one has to choose between
topical care or new skin grafts. Regenerated Integra is an inherently
healthy “naked dermis” which is effectively closing native tissues underneath. With some basic hygienic care, it can remain
open like any other wound. Left ungrafted, regenerated matrix either will
or will not epithelialize from the margins. In select circumstances in
healthy patients with smaller wounds, open care and natural epithelialization can
be opted in lieu of operative skin grafts 52. It must be remembered that regenerated
Integra is a mesodermal structure, and until epithelialized, some kind of
active care will always be required.
Delayed epithelialization and extended care seem to be quite acceptable
to most patients because the grafts are usually more healed than not, residual
open areas tend to be small, and the patients are already much improved.
Ancillary therapies. The nominal
reconstruction of Integra and one simple skin graft (outcome type 1a) is the
norm in young healthy patients with trauma wounds. In these study patients, only 22% had this
outcome. Another 10% required prolonged
topical care until the first skin graft was fully healed. While various non-specific wound dressings were
used, what distinguished this group and others was that some additional
deliberate intervention was done to promote reepithelialization. Used for their pro-proliferative wound
stimulatory effects, platelet-derived growth factor (PDGF, recombinant human
PDGF-BB, becaplermin, Regranex®, Ortho-McNeil,
Secondary procedures. Secondary surgery
means using second skin grafts, small flaps, or more Integra to complete a
reconstruction where the initial skin grafts did not take and could not be
coaxed to epithelialize. Most secondary
surgery was supplemental to the original reconstruction in patients doing well
rather than a bailout from the original plan in patients doing poorly. There was no consistency about when to do
another procedure. Whenever it became
clear that the current situation would not improve any further with topical
care only, the next procedure was done.
The same judgments were applied to the few patients doing poorly who
required amputation. (Cases 4, 10).
Planned second Integra. If an Integra reconstruction does mostly well, but there
are some unhealed areas, a second piece of Integra can complete closure of
those areas. There are also circumstances in which using multiple
sequential pieces of Integra is part of the strategic treatment plan.
Situations which warrant this include: using Integra as a long duration
artificial skin, replacing a fresh piece before silicone separates on the first
piece (case 16); maintaining uninterrupted coverage while waiting for
tangential histogenesis to bridge a gap (cases 3, 16); needing a thicker
multiple layer of regenerated tissue (cases 20, 25).
Long term management. Unlike normal
scars, for which maturation and related care can become foremost issues,
regenerated Integra is a nearly mature, mechanically compliant, esthetically
acceptable tissue by the time that skin grafts are placed and healed. It can be injured by trauma (case 22), but after
about 3 months, the new skin seems to be very durable. Simple protective wraps and continued edema
control were recommended for several months in most of these patients, but
because they were doing well, many did not follow up consistently. While the material might be somewhat resistant to the effects of recurrent
inflammatory and ulcerative disorders, this cannot be counted on. Continued management of underlying diseases
and risks is mandatory for the general health of the patient and the continued
health of the reconstruction.
Complications and problems. With proper wound
preparation, excision of the wound, and graft fixation, complication rates
should be low. Acute hematomas and loss
of adhesion due to motion are avoidable and can be easily managed by
evacuation, better fixation, and a new piece of Integra if needed. As regeneration progresses, the silicone can
be ejected before skin grafts are placed.
If that occurs, the open Integra can be managed by customary daily
hygiene and dressings until skin grafts can be placed. This will not jeopardize whether the wound
heals, but it does risk getting some inflammation and scar. However, as long as primary disease and
inflammation are under control, exposed structures are closed, and the original
wound is healed under the Integra, what happens on the superficial surface
becomes of lesser concern. Occasionally,
silicone separates in limited areas, resulting in blisters with turbid milky
exudates. Whether due to foreign body
reaction against the silicone or something else, they are not accompanied by
pain, erythema, nor destruction of the regenerated matrix. These sterile abscesses have been managed by
excising silicone wherever the problem is, and instituting daily care. Skin grafts typically do take in these areas. True infection, manifested as intense
inflammation, pain, suppuration, and loss of the material, occurred in only one
patient in this series, and it should be avoidable with good wound preparation
and excision. The management of lost or
delayed skin grafts is discussed above.
Open Integra. Loss of silicone or overgrafts is not the preferred
pathway, but it is no catastrophe, and the reconstructed new dermis can be
safely managed without a cover, by routine topical hygiene and dressings.
A consistent observation is that even when regenerated Integra remains
unepithelialized, the wound and periwound tissues remain free of inflammation,
pain, active necrosis and ulceration, and any other evidence of the original
problem (as long as underlying diseases are also adequately treated).
Unepithelialized “naked dermis” Integra is therefore therapeutic, far more
tolerable to patients than the original wound was, and sometimes even perfectly
acceptable.
Failed Integra. Applied to chronic
and pathological wounds, Integra usually succeeds, but active disease and
altered wound physiology mean that there is always some potential to fail.
While instances of failure were not many, there were several different
mechanisms. (1) “Gangrene” of the matrix. When Integra covers
non-viable tissues it turns black, a sure sign of residual undebrided eschar or
of extreme arterial insufficiency. (2) Failure to regenerate. The
matrix can persist as is, without evidence of histogenesis. Usually just
patchy, this seems to correlate with general debility or advanced
illness. (3) Early lysis or ulceration. Seemingly regenerated
matrix can suddenly ulcerate or involute, either before or soon after skin
grafting. Accompanied by new ulcers in surrounding native skin, this is a
dependable marker of underlying disease flare-up, typically immunopathic or
hematological disorders (Integra may be more tolerant of disease recurrence
than native tissues, but it is not invulnerable). (4) Failure to accept
or support skin grafts. Apparent take and then dissolution of skin grafts
has been observed by many practitioners. Second skin grafts usually
succeed, but if the Integra remains persistently open, it can be managed as
discussed above. This problem seems to
have occurred more in younger patients, less than 40 years old, and particular
reasons for the problem have not been deduced. (5) Conversion to a
conventional wound with inflammatory healing. This is a combination of
the above situations. If grafts do not adhere and disease takes over, the
open Integra eventually reverts to an ordinary wound. (6) Late
ulceration. Integra which has completely healed might reulcerate if
disease or maintenance care get out of control. If it already healed
once, it should be easy to get an incipient new lesion rehealed with good
hygiene, edema control, and anti-inflammatory and disease specific
therapies. In all of these situations, the problem is not inherent to the
matrix, but reflects problems with disease and patient management. When
these events do occur, the following should be done: reestablish good
daily wound care; reassess patient and
disease status; intensify treatment of
underlying disease if needed; check to
make sure that arterial vascular status has not changed during the course of
treatment; perform further debridement
as needed; rethink the overall treatment
plan. When wound and disease are back
under control, try again for closure, with new Integra or by other means
depending on the new plans.
Logistics. Unless a patient’s
underlying disease warrants inpatient care, all management is outpatient. Integra and skin grafts are all placed in the
operating room, with follow-up care in office or clinic. There is
sufficient latitude in the timing of the skin grafts to accommodate the
realities of scheduling. Many patients can continue their daily affairs
at home, with restrictions on activity, leg elevation, and ambulation based on
individual circumstances. When Integra
is used for trauma or elective reconstruction in young healthy people,
reconstruction is usually complete within 2 – 4 months, but times will be longer
for problem wound closure.
It is most important to realize
that, when used for chronic and pathological wounds, the cumulative time
required to complete an Integra reconstruction is anything but trivial. Using
Integra is neither difficult nor arcane, and 4-6 weeks of matrix regeneration
is not long, yet until the last square millimeter is epithelialized, active
care must continue. For chronic and
pathological wounds, treatment averages 5-6 months for most diagnoses, and as
much as 10 months for radiation and immunopathic disorders. Physicians
who do not regularly treat chronic wounds must appreciate these times and not
become anxious nor lose interest. The clinical process is simply
mirroring the biological dynamics of its regeneration. Normal inflammatory
wound healing works quickly, over days to weeks, and it then leaves a wake of
scar related complications that may require months or years of disability and
future care. Integra regeneration and related care occupies the middle
ground, measured in weeks to months. However, once it is healed, it
rarely needs further attention nor late revision. These treatment intervals may seem long to
physicians anxious to see good results, but they are accepted by most patients
because (1) the ulcer was present for months or years, (2) they are accustomed
to needing daily care, (3) once Integra is placed, symptoms and progressive
disease resolve, so function, lifestyle, and peace of mind are usually
improved, (4) most of the prolonged care is for small unepithelialized but
otherwise stable areas, and patients have long since returned to otherwise
normal healthy activities, and (5) Integra is succeeding where all else had
failed.
Caveats and
contraindications. As noted, the number of patients treated
with Integra in 6 years was a small fraction of the investigator’s total
experience. Many patients with similar
problems were treated by topical care or conventional procedures. There are no formal contraindications to
using Integra. It is categorically safe,
and even if treatment plans change, it is always dependable as a good interim
artificial skin in advance of any other reconstruction. Extreme arterial insufficiency was the one
single predictable physiological barrier to success, but Integra was also the
salvation of many arterial wounds and limbs that could not have been managed by
conventional means. Diabetic plantar
ulceration was also a poor place to use Integra, but for psychosocial rather
than physiological reasons (and for similar reasons, Integra is not well suited
to managing pelvic pressure ulcers). The main reason not to use Integra
is that a problem can be solved more expeditiously by conventional means.
Relative indications for Integra are presented above. The inverse is
true, if those conditions do not exist, Integra is not necessary. If
underlying structures are not exposed, if disease and risks are easily or fully
controlled, if dependable flaps are present, if prior conventional procedures
were uncomplicated, if a preliminary period of observation and topical care
shows that the wound is wound healing competent, then conventional management
and surgery should be done if that is what is best. All decisions should
be predicated on the goals of controlling disease and symptoms, healing the wound,
doing so as quickly and efficiently as possible with minimized costs and
resource utilization, all while preserving function and lifestyle.
Whatever treatment can be anticipated to best fulfill these goals should be
selected. The great majority of wounds are best managed by ordinary
means. Integra is used for those problems, large or small, life or limb
threatening, complex, pathological, refractory, and therapeutically challenging,
for which customary methods of care have not or will not work
Composite
Results
Costs of care were not directly
measured, but the inpatient-outpatient rates are an interesting reflection of
their times and the success of a new product.
The decline of the inpatient rate to zero reflects several factors: (1) increasing familiarity of the
investigator with Integra and its capabilities;
(2) increasing support for outpatient clinic and home health wound
services at the facilities where the investigator practices; (3) concurrent socioeconomic changes in the
delivery and payment of medical services in the United States driving care away
from the hospital. Integra is clearly a
product well suited to taking care of complex problems as an outpatient. Given the prolonged failed care that these
patients had prior to Integra or would have had absent Integra, and given the
success and outpatient nature of the care, the cost of an Integra
reconstruction for a chronic or pathological wound is assumed to be favorable.
Tables 3 show that 71 % of
patients (Group 1) had complete success, and 20% (Group 2) had partial
success. However, the way that these
results were tallied and reported skews the apparent results toward failure,
that is, tangible results were actually better.
For example, one rheumatoid patient died before the skin grafts were
completely healed, so she was assigned to group 2b, but Integra had clearly
performed well with dramatic improvements in the patient and all wounds. Case study 10 was likewise classified a
partial success (type 2a, group 2x) simply because a small secondary procedure
was needed, but the ability of Integra to close a flexor tendon and salvage a
finger in an atherosclerotic hand is a remarkable success. In one of the neuropathic diabetic patients,
overt non-compliance resulted in re-injury to the foot that necessitated
amputation. The Integra was inherently a
complete success, but because it did not contribute anything meaningful to the
final outcome, it was categorized as a Group 3 failure. In one of the arteriopathic patients, Integra
likewise performed perfectly on large leg wounds, but subsequent foot necrosis before
the reconstruction was healed necessitated amputation, consigning this case as
well to the Group 3 failures.
In retrospect, 7 patients with
extreme arterial insufficiency or diabetic plantar ulcers should be considered
poorly selected. The remaining 96
patients had criteria having a chance of success. Category 1 successes were then 76% of that
group. If Group 1, Group 2x, and half of
Group 2y are taken as those who had excellent ultimate outcomes, substantial or
complete success, even if touch-up procedures were needed, then successful use
of Integra for these patients is 92%.
Integra’s inherent performance is predictably good, it’s ability to
induce its relevant biological effects virtually guaranteed. In healthier patients with traumatic or
elective wounds, Integra should succeed 100% of the time. Under those healthy circumstances, any
failure should be considered a consequence of poor timing, unrecognized occult
diseases, or inadequacies of wound preparation and excision, graft fixation, or
overall management. For chronic and
pathological wounds, failures can result from the same deficiencies, but also
from poor patient selection, extreme arterial insufficiency, and inadequate
diagnosis or control of disease. These
problems are all extrinsic to the inherent performance of the material, and for
problem wounds, Integra is apt to outperform conventional repairs, grafts, and
flaps using living autogenous resources.
SUMMARY
Integra Dermal Regeneration
Template is a unique surgical implant that functions first as a high quality
artificial skin, and then becomes the agent of dermal regeneration. Its use is a type of in situ tissue engineering. It has many indications for the closure of
traumatic and surgical wounds and for elective reconstructive surgery and the
regulation of scar. This study
demonstrates its utility or superiority for the management of chronic and
pathological wounds. It achieves good
results – healed wounds – in situations where conventional wound closure
options have failed or amputations are threatened. Unlike topical care alone, Integra can
control wound conditions, inflammation, and pathergy, allowing a wound to
recover and regenerate at its own rate without further jeopardy. Unlike skin grafts, it is not alive, so it
does not risk loss due to necrosis, making it ideal for circumstances in which
ischemia and residual pathology threaten a graft. It’s ability to close essential visceral and
skeletal structures generally equals conventional flaps, and it is safer and
more versatile than many flaps, because it works when and where flaps are
unavailable, contraindicated, or uncertain to succeed. In this investigator’s practice, it has
supplanted many conventional wound closure procedures. The newly regenerated skin, having certain
embryonic characteristics, seems to be resistant to the effects of recurrent
disease such as rheumatoid panniculitis and venous vasculitis. Integra has no donor sites, and it incurs no
risk to the patient.
There do not seem to be any
inherent deficiencies in Integra’s ability to induce regenerative histogenesis
in the matrix. Absent surgical faults
and inadequate wound preparation, failures and problems can be attributed only
to severity and recurrence of disease or injury. Severe arterial insufficiency (ankle-brachial
indices approximately 0.3 or less) and non-compliant diabetic patients with
plantar wounds were the two consistent causes of failure. Nevertheless, Integra was the means of
success for many arteriopathic ulcers.
For most other patients in other categories of disease, Integra
succeeded where all prior attempts to treat had failed. Even when technical success was only partial,
where Integra did not fully epithelialize, clinical success was still good,
because wound closure was then completed with only minor further intervention,
and because the open Integra was always better than the original wound. Composite results out of 103 patients were
71% complete success and 20% partial success.
If those patients now understood to be poorly selected are excluded,
results were 92 % substantial or complete success.
Integra is a staged process, and
several months are required to complete a reconstruction. However, this is acceptable to patients
because ulceration is arrested, symptoms are relieved, care is simplified, and
recuperation and preservation of lifestyle are facilitated. Patients with chronic wounds are therefore
willing to bear the time required to complete an Integra reconstruction,
especially because the necessary care can be strictly outpatient for nearly all
subjects. This makes Integra ideally
suited to the changing economics and sociology of contemporary medicine.
Integra’s ability to protect a wound,
control inflammation, suppress normal wound repair and scar, induce embryonic
histogenesis, conduct histogenesis across gaps, withstand future flare-ups of
disease, and do so with no risk to the patient is a combination of features
unparalleled among surgical and wound products.
As a method of in situ tissue engineering, this surgical device is a
genuinely new paradigm of wound repair.
It is not an alternative to flaps and grafts, but rather an equal
option, and all must be selected based on their own merits, indications, and
criteria. Integra’s biological
properties, its safety profile, and its practicality make it the preferred
modality for a variety of problems. This
is especially true for chronic and pathological wounds, where conventional
repair, grafts, and flaps usually fail or are ineligible, but Integra
succeeds. After 30 years of development
and 10 years of clinical use, Integra is no longer a novelty product for burn
surgery. It is a versatile surgical tool
with unique properties and safety, and Integra ought to be adopted as a
preferred method of closing chronic and pathological wounds.
REFERENCES
1. Yannas
IV, Burke JF. Design of an artificial
skin. I. Basic design principles. J
Biomed Mater Res 14: 65-81, 1980.
2. Yannas
IV, Burke JF,
3. Yannas
IV. Studies on the biological activity
of the dermal regeneration template. Wound
Repair Regen 6: 518-23, 1998.
4. Burke
JF, Yanna IVs, Quinby, Jr WC, et al.
Successful use of a physiologically acceptable artificial skin in the
treatment of extensive burn injury. Ann
Surg 194: 413–28, 1981.
5. Heimbach
D, Luterman A, Burke J, et al.
Artificial dermis for major burns: a multi-center randomized clinical
trial. Ann Surg 208: 313–20,
1988.
6. Heimbach
DM, Warden GD, Luterman A, et al.
Multicenter postapproval clinical trial of Integra® dermal regeneration
template for burn treatment. J Burn
Care Rehabil 24: 42-48, 2003.
7.
8. Dostal
GH, Gamelli RL. Fetal wound
healing. Surg Gynecol Obstet 176:
299-306, 1993.
9. Bullard
KM,
10. Bertolami
CN. Glycosaminoglycan interactions in
early wound repair, in Hunt TK, Heppenstall RB, Pines E, et al. (eds.). Soft and Hard Tissue Repair: Biological
and Clinical Aspects.
11. Olutoye
OO, Barone EJ, Yager DR, et al.
Hyaluronic acid inhibits fetal platelet function: implications in
scarless healing. J Pediatr Surg
32: 1037-40, 1997.
12. Data on
file, Ethicon,
13. Ronca F,
Palmieri L, Panicucci P, et al.
Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis
Cartilage 6 SupplA: 14-21, 1998.
14. Hunt TK,
Knighton DR, Thakral KK, et al. Cellular
control of repair, in Hunt TK, Heppenstall RB, Pines E, et al. (eds.). Soft and Hard Tissue Repair: Biological and
Clinical Aspects.
15. Holbrook
KA, Smith LT. Ultrastructural aspects of
human skin during the embryonic, fetal, premature, neonatal, and adult periods
of life. Birth Defects 17: 9-38,
1981.
16. Gottlieb
ME. Modelling blood vessels: a
deterministic method with fractal structure based on physiological rules. Proceedings of the 12th
International Meeting, IEEE Engineering in Medicine and Biology Society, 1990.
17.
18.
19. Mozingo
DW, Ben-David K, Perrin KJ, et al.: Comparison of the biomechanical properties
of burns grafted with conventional split thickness skin vs. IntegraTM
artificial skin. Bostwick Burn and
Wound Symposium,
20. Orgill
DP, Straus FH 2nd, Lee RC. The use of
collagen-GAG membranes in reconstructive surgery. Ann NY Acad Sci 888: 233-48, 1999.
21. Chou TD,
Chen SL, Lee TW, et al. Reconstruction
of burn scar of the upper extremities with artificial skin. Plast Reconstr Surg 108: 378-84, 2001.
22. Dantzer
E, Queruel P, Salinier L, et al. Dermal
regeneration template for deep hand burns: clinical utility for both early
grafting and reconstructive surgery. Br
J Plast Surg 56: 764-74, 2003.
23. Moiemen
NS, Staiano JJ, Ojeh NO, et al.
Reconstructive surgery with a dermal regeneration template: clinical and
histologic study. Plast Reconstr Surg
108: 93-103, 2001.
24. Stern R,
McPherson M, Longaker MT. Histologic
study of artificial skin used in the treatment of full-thickness thermal
injury. J Burn Care Rehabil 11:
7-13, 1990.
25. Dabney
A, Voigt D,
26. Wiley DE,
Kowal-Vern A, Latenser BA. Successful
Application of Integra® in a Polytrauma Case.
Presented at John A. Boswick Burn and Wound Care Symposium,
27. Lorenz
C, Petracic A, Hohl HP, et al. Early
wound closure and early reconstruction. Experience with a dermal substitute in
a child with 60 per cent surface area burn.
Burns 23: 505-8, 1997.
28. Palao R,
Gomez P, Huguet P. Burned breast
reconstructive surgery with Integra dermal regeneration template. Br J Plast Surg 56: 252-9, 2003.
29. Kopp J,
30. Berger
A, Tanzella U, Machens HG, et al.
Administration of Integra on primary burn wounds and unstable secondary
scars. Chirurg 71: 558-63, 2000.
31. Fitton
AR, Drew P,
32. Soejima
K, Nozaki M, Sasaki K, et al.
Reconstruction of burn deformity using artificial dermis combined with
thin split-skin grafting. Burns
23: 501-4, 1997.
33. Gonyon
DL, Zenn MR. Simple approach to the
radiated scalp wound using INTEGRA skin substitute. Ann Plast Surg 50: 315-20, 2003.
34. Dantzer
E, Braye FM. Reconstructive surgery
using an artificial dermis (Integra): results with 39 grafts. Br J Plas Surg 54: 659-64, 2001.
35. Dantzer
E, Queruel P, Salinier L, et al.
Integra, a new surgical alternative for the treatment of massive burns.
Clinical evaluation of acute and reconstructive surgery: 39 cases. Ann Chir Plast Esthet 46: 173-89,
2001.
36. Wang JCY,
To EWH. Application of dermal substitute
(Integra) to donor site defect of forehead flap. Br J Plast Surg 53: 70-2, 2000.
37. Hunt JA,
Moisidis E, Haertsch P. Initial
experience of Integra in the treatment of post-burn anterior cervical neck
contracture. Br J Plast Surg 53:
652-8, 2000.
38. Giovannini
UM, Teot L. Aesthetic complex
reconstruction of the lower leg: application of a dermal substitute (Integra)
to an adipofascial flap. Br J Plast
Surg 55: 171-2, 2002.
39.
40. King
P. Artificial skin reduces nutritional
requirements in a severely burned child.
Burns 26: 501-3, 2000.
41. Milner
SM, Gulati S. Salvage of a Patient with
Abdominal Wall Dehiscence and Necrotized Skin using Integra®. Presented at John A. Boswick Burn and Wound
Care Symposium,
42. Besner
GE, Klamar JE. Integra Artificial Skin
as a useful adjunct in the treatment of purpura fulminans. J Burn Care Rehabil 19: 324-9,
1998. .
43. Ryan CM,
Schoenfeld DA, Malloy M, et al. Use of
Intergra® Artificial Skin is associated with decreased length of stay for
severely injured adult burn survivors. J
Burn Care Rehabil 23: 311-7, 2002.
45. Loss M,
Wedler V, Kunzi W, et al. Artificial
skin, split-thickness autograft and cultured autologous keratinocytes combined
to treat a severe burn injury of 93% of TBSA
Burns 26 :644-52, 2000.
46. Demarest
GB, Resurrecion R, Lu S, et al.
Experience With Bilaminate Bioartificial Skin Substitute and Ultrathin
Skin Grafting in Non-Burn Soft-Tissue Wound Defects. Wounds 15: 250-56, 2003.
47. Suzuki
S, Matsuda K, Isshiki N, et al. Clinical
evaluation of a new bilayer "artificial skin" composed of collagen
sponge and silicone layer. Br J Plast
Surg 43: 47-54, 1990.
48. Larson
KW. Treatment of Necrotizing Fasciitis
Wounds with Integra® Dermal Regeneration Template. Presented at John A. Boswick Burn and Wound
Care Symposium,
49. Vazquez
Rueda F, Ayala Montoro J, Blanco Lopez F, et al. First results with Integra artificial skin in
the management of severe tissue defects in children. Cir Pediatr 14: 91-4, 2001.
50. Greenberg
JE, Falabella AF,
51. Gottlieb
ME. Lower Extremity Lymphedema -
Management by Total Dermatofasciectomy and Skin Reconstruction with
Integra®. Presented at John A. Boswick
Burn and Wound Care Symposium,
52. Prystowsky
JH, Siegel DM, Ascherman JA. Artificial
skin for closure and healing of wounds created by skin cancer excisions. Dermatol Surg 27: 648-53, 2001.
53. Soejima
K, Nozaki M, Sasaki K, et al. Treatment
of giant pigmented nevus using artificial dermis and a secondary skin graft
from the scalp. Ann Plast Surg
39: 489-94, 1997.
54. Thomas
WO, Rayburn S, Leblanc RT, et al.
Artificial skin in the treatment of a large congenital nevus. South Med J 94: 325-28, 2001.
55. Shermak
MA, Wong L, Inoue N, et al.
Reconstruction of complex cranial wounds with demineralized bone matrix
and bilayer artificial skin. J
Craniofac Surg 11: 224-31, 2000.
56. Yeong EK, Yang CC.
Chronic leg ulcers in Werner's syndrome.
Br J Plast Surg 57: 86-88, 2004.
ACKNOWLEDGEMENTS
Data compilation and manuscript preparation supported by a
grant from Ethicon,