|
THE PHYSICS
AND PATHOLOGY OF WOUNDS PART 1: THE WOUND AS A SYSTEM AND A CONTROLLED
MACHINE Marc E. Gottlieb, MD, FACS Revision
01-a, April 4, 2010, Copyright © 2010 |
|||
|
Preamble This is the first of a series of three presentations that will
explore the origins of intrinsic chronicity and wound healing failure in
chronic and pathological wounds. This
is Part 1, The Wound as a System and a Controlled Machine. It will explain the wound as a complex
system, including principles of control and non-linearity. It will explain the wound as a system in
the language and science of systems – Physics. It will explain why control is not only the
basis for all wound dynamics, but why this is crucial to the functions of the
healthy wound and why pathological wounds misbehave. In Part 2, Auto-Immunopathy and the
Intrinsic Disease of Wound Healing, we will go from a physics-engineering
perspective to a clinical-pathological one.
The general stroma, the auto-immune connective tissue disorders, and
the chronic wound will all equated through the principle of sustained chronic
inflammation leading to immune sensitization against stromal elements. In Part 3, Chronicity and the
Intrinsic Disease of Wound Healing, we will bring together the engineering
aspects of the wound as a controlled process and the clinico-pathological
aspects of intrinsic auto-immune wound chronicity to understand why chronic
wounds fail to heal. |
|||
|
|
|
||
|
The Physics and Pathology of Wounds Part 1: The Wound as a
System and a Controlled Machine Part 2: Auto-Immunopathy
and the Intrinsic Disease of Wound Healing Part 3: Chronicity and
the Physics of Wound Failure These presentations are titled “The Physics and Pathology of
Wounds”. Physics and Pathology might
seem like an odd juxtaposition, but it gets more peculiar. What does auto-immunity have to do with
dynamical chaos? What does wound
failure have to do with controlled machines?
If these associations seem incongruous and “anti-biological”, it is only
because conventional biosciences tend to focus on classical biology, cell
biology, biochemistry, and the discrete interactions between paired elements
within biosystems. Understanding and
analyzing complex systems, as engineers would do, is generally a foreign
concept in biology. Yet all biology,
large scale and small scale, is a conglomeration of complex systems. Understanding how the many elements in a
complex system inter-operate to determine the timewise or “dynamical”
behavior of that system depends on the science of systems and complexity, and
that science is within the domain of physics. The overall purpose of these presentations is to explain a
theory and a set of observations and hypotheses about why certain chronic
wounds will not heal. The thesis
concerns the “intrinsification” of the wound due to the onset of stromal
auto-immunization and the appearance of chronic lymphoid inflammation. This pathological state of chronic
auto-immunization against the wound must be understood in part by the
conventional bioscience discoveries of the relevant cells, chemicals, and
other players on the stage of injury, inflammation, and wound healing. However, conventional biosciences cannot
readily explain the persistent failures or incompetencies of these wounds,
even after all primary disease and injury have been relieved, and even after wound
healing promotional therapies have all been tried. The true understanding of these impaired
wounds and systems depends on a knowledge of complexity, non-linear dynamics,
population dynamics, and self-organization – i.e. physics. To understand how this all goes wrong, we
will start by looking at what happens in the complex wound system when it is
healing properly. As we will now see,
there is an orderly set of dynamics that governs the healing process. It is a reference-driven, feedback-regulated
process – a control system – that ensures the proper behavior and output of
the repair process. Control is common
and deliberate in technological and human engineered systems, and hence it is
easy to liken a control system to a machine.
However, control is also an inherent, innate, and obligatory state of
most biological systems, and without control, most biological systems would
extinguish. This is not only true for
wound healing, but the control loop of wound repair is easy to discern,
define, and describe. It is the basis
for understanding the physics of chaos, populations, and self-organization
which will be necessary to understand why lymphoid intrinsification of the
wound is so ill behaved. |
|||
|
|
|
||
|
There is a generic approach to wound treatment that is the same
as for any other condition in medicine where there are acute and chronic
phases of the illness. The first duty
of the clinician is to get active disease under control, usually with a fairly
standard set of therapies, to prevent its progression, avert jeopardy to life
and limb, and alleviate symptoms. Once
acute disease is controlled, you then begin managing subacute or long term
aspects of the illness, to either cure the problem or make it manageable for
the patient, usually with many discretionary choices to be made based on
patients' individual needs. So, for
instance, the ruptured colon gets a laparotomy and colostomy, and when the
patient is recovered, you make all of the other necessary choices based on the
primary diagnosis (diverticulitis versus trauma, etc). For patients in diabetic ketoacidosis and
hyperosmolar coma, they get insulin and fluid and electrolyte management, and
when they are recovered, you can start to plan their long term dietary and
pharmacological management. For wounds, we control acute disease, injury, thrombosis,
necrosis, inflammation, bioburden, edema, ischemia, etc., until the wound no
longer threatens the patient. The
physiology of wound healing is such that it isn’t going to heal anyway until
these things are controlled. Once they
are controlled, you then begin to pick and choose treatments that will get
the wound healed, or get it healed faster, or make it easier to live
with. The basic model of medical care
is the same for wounds as it is for anything else. For the colostomy patient above, the
discretionary planning is simple enough, based on primary disease and patient
status and wishes. Teaching him how to
use a colostomy bag or else restoring the continuity of the colon are easy
enough, and barring the occasional complication, they are predictably
effective. The diabetic patient will
have a variety of choices to make about diet, weight, exercise, and
medications. If the patient is
incompetent or non-compliant, treatment will fail, but the disease itself
remains inherently responsive to such therapies, and good diabetic management
is the norm for a cooperative patient.
So, for wounds, we apply the same principles of care, and what
happens? The first answer is that, yes, we certainly have lots of
successes. However, anyone who deals
with chronic and pathological wounds knows that, while the principles are
sound, actually getting some wounds to heal is not so easy. For some wounds, in spite of all due
diligence and wile, in spite of 82 different therapies you have tried,
ranging from voodoo and shamanism through expensive operations, and
everything in between, some of those wounds just will not budge. They are stuck where they are, mocking
every feeble effort you make to show them who’s boss. They refuse to heal even when gross
pathology and causative disease are fully controlled and acute active
ulceration and inflammation are fully subsided. Case 1, upper: This is a 76 year old woman with long standing
rheumatoid arthritis and a refractory ankle ulcer. The three images are over an interval of 18
months. The graphic curves show wound
size. An initial improvement early in
the course of treatment is typical of most patients – we can almost always
make improvements by instituting good care or doing some surgery. However, after those early gains, progress
and wound size level off.
Month-to-month there will be slight variations in size and appearance,
but no net gain over long intervals.
During this period of time, many technology based modalities and
surgical procedures were tried. Case 2, lower: This a 35 year old woman with sickle
disease and a refractory ankle ulcer.
The three images are also over an interval of 18 months. All of the comments about the first case
apply here. Despite persistent care
and multi-modality approaches to care, the wound just will not
cooperate. Yet in both cases, they
look mostly like they should be healing.
The wound surfaces are somewhat altered from normal, but there is no
gross inflammation, i.e. no edema, erythema, active ulceration. The periwound is healthy. Why would these wounds remain so refractory
when all of the features and history, natural and therapeutic, suggest that
they should have healed months ago? It is the explicit purpose of this series of three lectures to
show that there is a reason for this frustrating and confounding
behavior. It is a real world physical
reason. However, the answer does not
lie with any single gene or protein or receptor or cell or organelle or
whatever. It is a reason that relates
to the inter-operations of all of them.
Understanding them means understanding the generic principles of how
inter-operating systems work, and that means understanding their relevant physics; In considering these problematic wounds, there is a reason: these wounds go back and forth but get no
better; they cannot spontaneously
climb out of this attractor; multiple
therapeutics are often of no benefit; this
adverse behavior is independent of the primary pathology. Furthermore, these reasons cannot be
understood: by looking at any
individual cell or chemical or gene, nor by analysis of any
dependent-vs-independent experiment, nor by any other “conventional
bioscience” type of experiment, nor by any type of randomized or other
clinical controlled trial. Remember,
we are not talking about wounds that are sick and getting worse due to active
disease. When a wound is sick, you
control the underlying disease, and things then either heal or at least
remain stable. No, we are talking
about those in which you have controlled all adverse conditions, and it
should be healing, and it even looks perfectly healthy as though it should be
healing, and you are frustrated because you cannot discern any reason why it
isn’t healing – but it isn’t. Over the
course of these lectures, the reasons will be elucidated. We start by looking at the most central
physics concept that governs these behaviors: non-linearity and control. |
|||
|
|
|
||
|
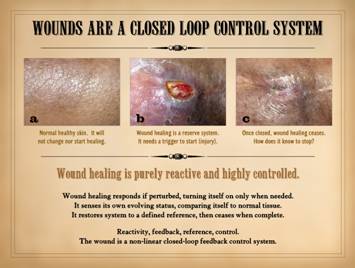
Wounds are a closed loop control system. For the wound which otherwise seems healthy
and as though it should be healing, this concept is the starting point for
understanding all the other aspects of its apparent misbehavior. We start by looking at an example which at
first might seem trivial and obvious to the point of absurdity, but once you
genuinely understand it, everything else follows. The middle photo shows
a wound. On the right it is healed, the same wound. The left
image is from the same patient, at the same time. It is normal skin. It is not healing. “Of course it’s not! . . . So? . . .” you
might say. But think about it. The wound started to heal. How did the wound know when to start
healing? How is it that it stays
confined to the injury, rather than triggering a process of angiogenesis and
fibroplasia throughout the body? Why
doesn’t the normal skin in the left just start healing for no good
reason? “Simple”, you say, “because it
wasn’t injured.” So, it is a reserve
system, but how is that injury tells it to start healing, but otherwise it
knows enough to stay asleep? Once it
starts healing, how does it know when to stop? The obvious is not necessarily so trivial,
and profound principles come in the simplest of observations. Biological systems almost all have promoters and
inhibitors. Some agent tries to make
you to do something, and a counter-agent puts the brakes on. If biological systems did not have such
promoter-inhibitor balances, they would get out of bounds, racing ahead,
exceeding their capacity, overwhelming themselves or their contingent
systems, or else extinguishing, failing to achieve or sustain necessary
metabolic functions. Biological
systems and life are entirely dependent on the ability of promoter-inhibitor
agents to keep a system within bounds, to keep it from over-reacting or
under-reacting. The ability to react
and counter-react, to find the healthy “center”, to avoid overwhelming the
system or dying out, that is what is meant by control. Nearly all biological processes, and nearly
all healthy “homeostatic” states of those processes are being regulated,
controlled, to maintain their desired state.
Of course, when we say “desired”, we are ascribing teleological
intentions to the system. In reality
these systems find their “centers” because of dynamical and thermodynamical
principles that govern how all complex chemical and cellular processes must
behave. Conventional bioscientists are used to thinking about all of the
biochemicals and cell structures which are the tangible promoters and inhibitors
of such systems. However, when it
comes time to understand how all of these chemicals get together and find
their balance, it is not possible to do so by chemistry principles
alone. Physics and engineering
principles are required to understand the dynamics of regulated systems, how
they behave and react over time and under the influence of many mutual
inter-operating factors. Once you
understand the principles by which regulated control systems operate, it is
easy to see how the normal wound heals, but also how it goes wrong and how it
might be treated when it is wrong. As a system, the following are crucial properties of the
wound: Wound healing is purely reactive
and highly controlled. Wound healing
responds if perturbed, turning on only when needed. It senses its own evolving status,
comparing itself to a reference – normal tissue. It restores the perturbed system to that
defined reference, then ceases when complete.
The “nuts-and-bolts” of how it senses these things and actuates a
response is where conventional biology comes in, but the dynamical responses
and behaviors of the system are “device-independent”, unconcerned with the
details of each molecule or organelle.
The reactivity and responses of the system depend on feedback, a reference,
and control. These conditions qualify
the system as “non-linear” meaning that it is self-dependent, its future
value or state being a function of its current value or state (unlike a
“linear” function as defined by algebra and calculus where the system value
would be a function of an independent parameter). In short, the wound is a non-linear
closed-loop feedback control system. |
|||
|
|
|
||
|
To understand the dynamics of misbehaving wounds, here are
examples of variances or faults – unregulated, broken, atypical attractor –
in the operations of the wound healing “machine”. Upper left: No healing.
A 6 month buttock ulcer in a 44 year old woman with severe rheumatoid
arthritis on cyclophosphamide. There
is no evidence of wound healing. There
is a slight blush of angiogenesis, but the yellow color of the adipose is
still obvious, and the lobular architecture of the fat is 100% unaltered
because there is no fibroplasia whatsoever.
Likewise there is no epithelialization. Yet there is also no edema, erythema,
induration, active ulceration, etc.
The wound is not acutely pathological, but it is not healing. This is one of the most dramatic examples
of an arrested wound module that you will ever see. Upper right: No healing.
This image shows the tail end of a wound closure or reconstruction
using a collagen-gag regenerative matrix (Integra® dermal regeneration
matrix). The epidermis on top has not
yet grown to confluence, leaving behind open areas of the regenerated
dermis. In the clinical or vernacular
sense, this is still a wound, and yes, it is healing. The intent here is not to “split hairs”,
but from a physiological and dynamical point of view, this is not the wound
module functioning. Angiocytes and
fibroblasts are reassembling a dermal analogue according to the events of embryonic
dermatogenesis, and thus they are using a set of dynamics and self-assembly
sequences which share certain features yet are distinctly different than
normal healing. Clinically, this
“wound” has a happy favorable status, but it is nonetheless on an alternate
or atypical attractor compared to normal wound healing. Lower left: Improper healing. This is a pyogenic granuloma (more about
this on slide 28). It represents
healthy and qualitatively normal wound healing gone to quantitative
excess. The problem is one of
unregulated proliferation because the normal controls or regulators in the
system have gone awry. Specifically,
the system load (angiocytes and “granulation tissue”) is being spoofed by an
interloping controller (macrophages in the bandages) operating in parallel to
the normal controller (macrophages in the wound). Lower right: Excess healing. This is a typical keloid, a pathological
form of scar hypertrophy that occurs for non-physiological reasons. Compare this to hypertrophic and contracted
scar that occurs in response to the mechanical forces applied (tension, such
as after an injury across a flexion surface of a joint). Such reactive contractures are clinically
problematic, but they are a physiologically correct response to the
mechanical forces applied – from a fibroblast’s point of view, a proper
response to an improper event.
Reactive hypertrophy from mechanical metaplasia is also a fully
regulated, closed-loop controlled process, just like normal healing, and it
will cease when forces in the tissues are balanced. In comparison, the keloid has no
discernible correct reason for being the way that it is. It is a condition of unregulated
fibroplasia, in which the fibroblasts are either unresponsive to controls or
else they are being controlled by abnormal stimuli that mask or overwhelm the
normal controllers. Right: Chaotic.
This case is comparable to those shown on slide 2. Over a 1 month and then a 4 month interval,
there is no net change. It is in a
chaotic “orbit”, a state that has a technical meaning from mathematics and
physics, which will be explained further in this Part and especially in Part
3 of this series. The whole purpose of
this series of presentations is to explain why wounds which behave this way
have found a state of relative dynamical and thermodynamical stability from
which they cannot easily escape. From a clinical point of view, most of these behaviors are
undesirable and contrary to health or normal unfettered life, From a biological point of view, these
examples would generally be seen as pathological variances from normal
physiology. This is where a classical
bioscience perspective diverges from a mathematics and physical sciences
point of view: as unnatural or
undesirable as these states are from
the medical perspective, they are nonetheless the standard permissible
behaviors (dynamics) of controlled and complex non-linear systems, including the
wound. |
|||
|
|
|
||
|
Within the past decade, at the very turn of the 21st
century, the human genome was first sequenced. Before and much more ever since, the
complete DNA sequence for many species has been read. There was a general sense – very naive –
that all things in human pathology and clinical medicine would now be
understood and fixed by some gene therapy.
That of course makes no more sense than the pundits who a century
earlier thought that all disease was caused by a microbe and could be cured
by their elimination. The fact that
only about 30,000 genes were found in the human genome was also a surprise –
way too few to explain the myriad and countless elements of biology that are
already well understood. There are
many points of extraordinary naivete in the initial premise of “discover all
the genes and all will be revealed”. First,
it turns out that there is extra non-gene DNA in the genome, and that does
something too, so regulators on genes as much as genes themselves might have
something to do with the actual living biology. Then, the genes only encode for a few
structural proteins (e.g. collagen), and the rest (enzymes) are catalysts and
process regulators for conventional chemistry. Gene expression is through additional
self-assembling intermediaries (e.g. RNA), and the results of proteomic
activity are the many thousands of small biochemicals that float in the
system. After that, interactions
between chemicals makes other chemicals, and don’t forget their degradation
products which also have bioactivity.
Add to that mix the effects of simple ionic, acid-base, and inorganic
chemistry, and by the time you are done, the number of unique molecular
species in the body with a biologically relevant role numbers in at least the
hundreds of thousands, and more likely the millions. Just knowing the names and spelling of
30,000 genes is necessary but remotely far from sufficient to understand the
workings and failings of the human body. When you look at how 30 thousand or 30 million agents
inter-operate, you are looking at a “complex system”. In fact a system of just 1 or 2 agents can
have complex behaviors, and from the point of view of mathematics and
physics, any system of 3 or more agents is ipso facto complex. What actually do we mean by “complexity”? There is no exact definition. In part, complexity has the same meaning
that it does in vernacular speech – intricate, detailed, and highly textured,
structured, and interconnected.
Technically, it also means that a system is non-analytical, i.e. it
cannot be described by the functional relationships of algebra and
calculus. The study of complexity and
complex systems is rolled up in the physics subtopic of non-linear
dynamics. Some historical perspective
is needed here. Until the 21st
century, biosciences were wrapped up in the very important (and well-funded)
explorations-on-the-frontier of biological chemistry (including cell biology). These explorations focused on
characterizing the structure and core chemistry of biological chemicals, and
characterizing their simple one-versus-another reactions and interactions in
isolated laboratory circumstances.
Evaluating complex in vivo activities, especially those involving
multiple interacting elements that cannot be isolated into one-versus-another
experiments were deprecated as unworthy of serious study. There was the doctrine in the biosciences
that all studies must be conducted as one-versus-another with everything else
held static. To be fair, there was a
doctrine in all of the natural sciences that all events must be characterized
as a properly defined differential equation of one variable versus another. To do otherwise, to “solve” problems with
iterations, interpolations, and piecewise approximations were the cheap
floozies of the mathematical sciences, prostitutes of necessity to be
indulged by engineers who needed their services, but not the kinds of
algebraically respectable women you might take home to your mom the math
professor. These comments are in no way a derogation of the science of the
20th century – obviously extraordinary amazing mind-boggling
discoveries and applications were achieved.
But many questions about the natural world could not be solved with 20th
century science. Those who derided any
effort to study complex system-wide interactions were simply acknowledging
the fact that there were no tools at the time to study such systems, so why
waste your time? Trying to solve or
model the overall integrated operations of interconnected complex systems was
beyond the analytical or computational means of those times. Systems science was simply not possible
until the 1970’s or 1980’s. What
changed was the advent of theoretical foundations for non-linearity, the
creation of computational technologies that can implement the theories,
foundational concepts in complex modeling as well as the acceptance of
sophisticated piecewise approximators such as the finite element method, and
the education of a new generation of scientists not shackled to old
doctrine. The history and psychology
behind this is explored further on slide 18.
Sadly, systems science since the 1980’s has caught up slowly – barely
at all – in the biological sciences.
Now that we have elucidated the genome, we still know hardly anything
about how those 30,000 genes do their business and lead to disease. Some of those answers will come from the
methods of conventional 20th century biology, studying each gene
per se or its immediate protein product.
However, many answers will not come unless the complex interconnected
multi-factorial systems that these genes encode are studied as such – complex
systems – using the mathematics and science of complexity and systems. The study of systems and complexity has
come to the biosciences more slowly than for the physical sciences, but it is
starting: “A field known as Systems
Biology is emerging, from roots in the molecular biology and genomic biology
revolutions – the succession of which has led biomedical scientists to
recognize that living systems can be studied not only in terms of their
mechanistic, molecular-level components but also in terms of many of them
simultaneously.” (Ideker T, Winslow LR, Lauffenburger DA. Bioengineering
and systems biology. Ann Biomed Eng. 34(2):257-64 and 34(7):1226-33, 2006.) So, welcome to the Century of the System. For the past 150 years, we have been trying
to explain everything based on biochemistry and 19th concepts of
science. Now we are in the 21st
century with new methods and new discoveries to answer both new questions and
the unanswered questions of prior eras.
Old science: Physiology
of the 20th century, an age of biochemical discovery, was grounded in
chemistry. It focused on one-on-one
reactions and kinetics between any two chemicals or bio-parameters. It promulgated “homeostasis”, predicated on
chemical concepts of reaction equilibrium. Physiology research depended on linear
models of dependent-versus-independent parameters in an otherwise invariant
environment. For wounds, this classic
style of research has characterized hundreds or thousands of cellular and
chemical interactions. But biological
systems do not really work that way.
They are built from many simple elements of that nature, but they have
MANY levels of inter-operation, defining them as non-linear and
multi-control. The mutual integrated
behavior of such systems cannot be assessed analytically by simple balanced
equations. New science: How do 30,000 gene products and a bazillion
derivative chemicals interoperate to make Life? To understand complex systems, principles
are needed from the sciences of complexity, the principles of non-linear
dynamics and control science. The
wound is a paradigm of a complex n-body non-linear multi-control system. Its physiological behaviors and
pathological misbehaviors can be readily explained when wounds are understood
as a System, rather than as just a collection of dual-element linear
interactions. Accurate understanding
of its many behaviors must start with a meaningful model of the whole
machine, the System, and not just its chemistry-oriented individual
components. As a controlled system,
the quintessential intrinsic machinery of wound healing – the “Wound Module”
of proliferative post-inflammatory wound repair – functions as just a single
control loop. This presentation, Part
1, will introduce basic concepts of
closed loop control and then characterize the Main Control Loop of normal
physiological wound repair. The wound is a dynamical system –
meaning that it evolves in time. That
is what defines a dynamical system – its dependence on and evolution in
time. Many problems in physiology and
pathology are dynamical, even if bioscientists are not used to thinking of
them that way. However, every time you
invoke the word “pathogenesis” you are making that implication. Of course, for the sake of daily medical
practice, many diseases need not be thought of that way. For many clinical problems, an issue may
seem static without variance, or else it may evolve in time in a trivial
one-way smooth slide from here (healthy) to there (sick) without any
irregularity. For example, hyperparathyroidism
could be seen as being a relatively static hyperactivity of parathyroid cells
and elevation of parathormone. The progressive
state or evolution of the parathyroid glands or cells per se is not of much
relevance to its complications and clinical care. How hyperparathyroidism then leads to bone
dystrophy and possible renal failure is much more clearly a dynamical event
that evolves in time. However, if
untreated, the states of bone and kidney are likely to get progressively
worse in a generally “linear” or smooth profile going steadily from good to
bad to worse, with no back-tracking nor ups-and-downs. If a parathyroid adenoma is removed, then
the problem is resolved, and further timewise misbehavior of the system will
not occur. Compare this to a patient who has
congestive heart failure, aortic stenosis, coronary artery disease, low
ejection fraction, emphysema, pulmonary hypertension, systemic hypertension,
and hypertensive nephropathy with less than half of normal glomerular
filtration, who then gets pneumonia or a broken hip or acute diverticulitis. It is the perfect recipe for mortal
disaster, yet such a patient can be managed and with good outcome. Good care and outcome is contingent on the
intensive care unit where moment-by-moment monitoring and treatment are
implemented. We do the constant
monitoring and treatments because the state of that patient can vary from
moment-to-moment. The dynamical nature
of the problem is intrinsically understood by all, even if most doctors have
never explicitly studied that situation in such engineering or mathematical
terms. Each time we take a set of
clinical measurements, we are assessing the state of the system. We “feedback” this information into the
system by comparing current values to target values, then calculating
corrections. Each time we then
implement a therapeutic correction, we are controlling some vital component of
the system, which hopefully responds by bringing the system back toward the
target values. What we have just
described is a feedback-regulated closed-loop control system. While this may sound like engineering
terminology, something designed into a machine, the reality is that human
engineered systems are simply doing what biological systems do naturally –
trying to regulate a system at a desired value. In normal healthy biology, ALL systems
are feedback regulated and controlled around some defined value. This is the origin of traditional concepts
of “homeostasis”. The reality is that
biological systems, from the folding and resonance of a single peptide
through the integrated functions of the cardiopulmonary system MUST stay
within certain physiological parameters if the host is to be healthy. If systems or parameters tend to wander out
of allowable bounds, or if they get kicked out by some perturbation, then
something else senses the variance and reacts to bring things back within
bounds. Thus, parathormone,
calcitonin, calcium, and phosphorus levels are all sensed, and parathyroid,
bone, and calcium metabolism all stay within healthy bounds. In contrast, the parathyroid adenoma has
become unresponsive to controls, ramping the rest of the system out of
bounds, an unhealthy state of pathology.
Every physiological system in the body, from control of heart and
respiratory rate, to maintaining the proper balance and trigger point of the
plasma protein thrombosis system, to generating the correct density of
capillaries within a tissue, to tracking an object with your eyeball and
pressing the correct button on your remote control without a tremor or a miss
– EVERY embryological and EVERY physiological system is feedback regulated
and controlled, and physiological parameters stay within healthy bounds. When the body can no longer regulate, when
parameters get out of bounds, that is pathology, that is illness. For the sake of this whole discussion,
when we say “wound”, we are referring to the physiological process of repair,
i.e. the wound healing system. Of
course, “wound” can mean many things, from the injury to the defect to the
repair process – one term for various interrelated concepts. The intended meaning usually should be
clear from the context, but to be explicit, much of the use here of the word
”wound” will refer to the wound process, the wound physiology, the repair
system that puts things back together after an injury or disease makes a
defect or triggers an inflammation-repair response. That said, the wound is a
system. When healthy, it operates to
correct an injury or defect in the body.
It gets back to a normal tissue architecture by following the pathway
of a closed-loop reference-driven feedback-regulated control system. When it is healthy and in bounds, it stays
in bounds with little activity or energy.
When rocked out of bounds by some perturbation (injury), it responds
to drive the system back to a stable restored tissue. This process is a sequence of integrated
events that occurs over time, and thus the wound is a dynamical system. Time, dynamics, control, stability – all of
these terms and concepts are mutually contingent and intertwined in a
properly functioning or healthy system, regardless whether we are talking
about the flight controls on a rocket, the regulation of heart rate and blood
pressure in the patient described above, or . . . the healing of a wound. |
|||
|
|
|
||
|
In order to draw the connections between the wound as a
biological entity and the wound as a physical system, we must first have a
basic descriptive explanation of the architecture-anatomy and sequence of
events in normal wound healing. In the
photographs on the right, a normal healthy wound goes through the natural
process of healing until it is closed, i.e. epithelialized. Histologically, on the left, all of the
reparative events taking place in the wound have a well organized and recognizable
anatomy, and each of the features seen microscopically correlates with
something that is happening or can be observed grossly. What is that anatomy and organization? What is the sequence, and how do we
recognize these events? The basic biology of wound healing can be epitomized in one
concept, the Wound Module of
post-inflammatory proliferative repair.
This term was coined by Dr. Thomas K. Hunt, San Francisco
surgeon-scientist and pre-eminent wound researcher of the latter 20th
century. It is the core anatomy and
physiology of wound healing, the same as bronchi and alveoli to the lung
doctor, the same as the myocardium and valves to the heart doctor. What you observe on physical examination of
the wound correlates with some distinctive event or element in the cellular
physiology of wound healing. It is the
orderly appearance, interaction, and assembly of these elements that
constitutes the wound module. While
the whole process has bazillions of individual chemicals and interactions
(the stuff of everyday laboratory wound research across the globe), the
process is conceptually quite streamlined and easy to abstract. In this short discussion, the process will
be reduced to 7 key items, 7 physiological events with 7 clinically
observable correlates, the quintessential “seven clinical signs of wound
healing”. 0 – Injury and
inflammation: Wound healing is a
reserve physiology, the wound module an ad hoc organ. They appear when injury disrupts the
integrity of the body. The body’s
response to any injury is inflammation.
Inflammation is the protective and destructive response that defends
the body during injury, then cleans up the debris, then initiates the healing
process. Without an initial injury and
then inflammation, wound healing is not there. However, the process is complex, because
while inflammation triggers the healing process, sustained inflammation also
suppresses healing. This is a way to
ensure that resources are not wasted, by delaying repair and not permitting
it to run fully until the field is sufficiently stabilized and cleaned
up. Recrudescence of injury and acute
neutrophilic inflammation will put wound healing down again. Injury and inflammation are the predicates
to healing. They get the process
going, but only as they themselves are leaving. If significant inflammation is present,
grossly or histologically, the wound remains in acute phases, and healing
does not appear. 1 –
Inflammation subsides: The first
sign of wound healing is that inflammation subsides. As an inhibitor of the wound module, high
levels of inflammation must wane before the wound module will
accelerate. Clinically, there will be
subsidence of erythema, edema, warmth and hyperemia, pain and tenderness, drainage,
necrosis, and other markers of injury and acute response. If this does not happen, the wound module
will not progress. If these changes do
subside, that is the harbinger of proliferative repair events. 2 –
Macrophages, eschar separation, and cytokines: Macrophages arrive in the wound as blood
borne monocytes. Inflammatory
mediators such as pdgf transform these cells into the macrophage. As acute inflammation and other leukocytes
clear out of the wound, these cells remain to do the keystone job in the
integrated inflammation-repair process.
Macrophages actually have two major roles in the wound. Their afferent
task is as phagocytic cells to remove debris.
Whatever is dead or damaged and needs to be cleared, they do it. (An ancillary role in this regard is to
present antigen to lymphocytes as part of immune recognition and defense
against xeno-pathogens, stuff that they find as they mop up. This function is tangential or irrelevant
to the wound module and normal wound healing per se. However, in chronic pathological wounds,
this becomes the basis of the auto-immunization which perpetuates wound
chronicity, which will be discussed at length in later sections.) Clinically, the afferent function of the
macrophage is recognized by eschar separation – dead stuff is cleaved from
the living stuff, and the dead stuff bit by bit falls off and
disappears. Their efferent task is to initiate the repair process. The local repair cells need something to
flip the switch to “on”, and it is the transformative and stimulatory
cytokines and growth factors made by the macrophages which do this. They include bfgf, pdgf, vegf, igf, and
others, all of which act to stimulate local vascular and fibrous cells. Clinically, the efferent effect of
macrophage wound stimulation is recognized because all of the subsequent
items on this list begin to appear. 3 – Ground
substance and mucus: The purpose of wound
healing and the wound module is to reconstitute a basic stroma that holds the
body together and provides a foundation for epithelial growth. Native stroma and repaired stroma have
collagen and other connective proteins as the structural matrix. However, early cells in the wound need a
place to live and do their thing as they make the new connective matrix. Architects and builders must create some
form of staging on which construction workers can stand, so that they can lay
the bricks and mortar, the stones and steel of some new building. Plasma proteins constitute the topmost
layer of the wound, where acute inflammatory cells do their work. Below that is a zone of glycosaminoglycans
(gag’s), ground substance, where the early repair cells, angiocytes and
fibroblasts, can live and do their job.
The aminoglycan layer is the construction staging. The gag’s are created by inflammatory and
arriving mesenchymal cells. One of the
earliest signs that the wound is entering the proliferative phase, clinically
it is recognized by mucus and light reflex on the wound. 4 –
“Granulation tissue” and angiogenesis:
This is the most obvious positive wound finding to naïve observers,
the red pebbly carpet of new blood vessels that appears, eventually covering
the entire surface in any wound that is properly healing. This tissue is new blood vessels forming in
the aminoglycan matrix. The angiocytes
that make the new vessels are being attracted from old vessels below by
angiogenic cytokines made by macrophages above. Vascular density is much higher than in
normal tissues, hence why it is so red.
Once these new vessels are established, they create the favorable
environment in which fibroplasia can then occur. 5 –
Histioblasts, fibroblasts, and fibroplasia:
Once angiocytes have formed vessels within the aminoglycan layer,
there is now an environment hospitable to other cells. The other cell which has a restorative function
is the histioblast-fibroblast. In this
presentation, “histioblasts” will refer to the earlier incarnation of these
cells, the uncommitted pluripotent stem or reserve cell line that will spawn
new fibroblasts when needed. The
“fibroblast” is the more mature version, making and embedding itself into the
new connective protein matrix. The
matrix starts as amorphous fibrillar collagen, and as it becomes denser and
more mature, it becomes more fibrous with its characteristic mechanical
properties. Clinically, thus us
observed as stiffness in the wound, less mechanical compliance. 6 –
Myofibroblasts and contraction:
Wound closure ultimately is defined by the restoration of an
epithelial boundary which sequesters the mesenchyme from the ambient
world. However, to lighten the load on
the epithelium, nature has another trick, wound contraction, which reduces
the size of the wound. To do this,
some fibroblasts develop muscle proteins and become contractile. The function of these myofibroblasts is to
ratchet the wound together: tug with the muscle proteins, then cement with
the connective proteins, then tug with the muscle proteins, then cement with
the connective proteins . . .
Clinically, this is recognized by in-curling of the wound edges,
smoothing of the wound contours, and progressive reduction in wound width and
size. 7 –
Epithelialization: Epithelialization that
separates insides from outsides is the final step. For epithelium to grow across the wound,
all other components of the wound module must be in place. Epithelium will only start to grow where
“granulation tissue” is in contact with the wound edges. Once the process starts, thin new epidermis
(or any epithelium) outgrows across the surface until the whole thing has
been “painted”, a process very easy to observe clinically. 8 – Maturation: The seven events and clinical signs of
wound healing and the wound module have now been witnessed: inflammation subsides >>
macrophages & eschar separation
>> ground substance &
mucus >> angiogenesis & granulation tissue >>
fibroblasts & fibroplasia
>> myofibroblasts &
contraction >> epithelialization. The wound is now nominally closed. However, wound healing is not over. The newly restored stroma is excessively
dense with new connective proteins and vessels, and the mechanics of the
tissue and functions of the epithelium are far from mature. Over a period of months or years, the new
scar will be reworked and remodeled back to something akin to natural dermis
or fascia. Those slow changes also
have their clinical observations, mainly improved color and compliance. This slide presented the general functions of the wound module
and what you will see clinically that correlates with these events. The next seven slides will focus on wound
anatomy, what you will see under the microscope, which likewise directly
correlates with wound module events and the 7 clinical signs of active wound
healing. These following slides are an
abbreviated version of a larger presentation on normal wound healing. You can read more and get the thorough
story on the Arimedica website: http://www.arimedica.com/content/integra%20histogenesis_gottlieb-me_v2003.htm http://www.arimedica.com/content/arimedica_integra%20histogenesis_gottlieb-me_v2003.pdf |
|||
|
|
|
||
|
On this slide, we will describe the anatomy of the wound. The wound module, with its constituent
cells, chemicals, structures, and interactions, is not just a jumbled mix,
not a tossed salad of neutrophils and macrophages, and stromal cells. It is highly structured, and each aspect of
that structure means something important to the health or morbidity of the
wound. Notice the order of discussion:
the last slide described wound module physiology and the correlated clinical
and physical exam features, and now this slide describes anatomy. This is backwards compared to how biology
and medicine are traditionally taught.
For example, it is much easier to fathom cardiac function once you
understand the structure of the chambers and valves. Why backwards for the wound? Because this is a dynamical system. Time defines a sequence of events, and
those events in turn define the resulting anatomy. In a sense, every active wound is an
embryological event in which the wound module is born, grows, and matures as
it fulfills its functions. It is much
easier to understand the anatomy once you understand the events which formed
them. The wound is structured vertically. Observed histologically, there are
distinctive strata, going from the surface down to the layer where all of
these events and effects give way to normal virgin native anatomy. This vertical anatomy of the wound reflects
timewise events and sequences. The
surface is happening now. The
fibroplasia layer deeper down started so many days ago. The various strata in between reflect the
timewise events described on slide 6.
Maintenance of these strata, and the separation of cells and
populations (acute inflammation and wound module) by time and vertical zone
are a crucial part of healthy wound physiology. When cells and strata start to become
intermixed, that is both cause and consequence of prolonged injury, delayed
healing, pathological events, chronicity, and refractoriness in the wound. Remember: in the normal healthy wound, the
physiology is a set of sequential events which leads to an anatomy of
vertical strata, all of which have correlated clinical findings, and all of
which becomes pathological when events and strata start to become chronic and
intermixed. On the left of this slide are two long vertical images, two
prototypical examples of completely healthy wounds healing properly (seen
with basic hematoxylin and eosin stain).
The center one is shown in detail via slices representing 6 major
strata of the healthy wound. All of this is taking place within a depth of just a few
millimeters (the depth will vary, greater or lesser, with location and the
circumstances of each wound). Zone 1 – Inflammatory or plasma
protein layer:
This is constituted of plasma proteins, leaked from vessels
underneath, serving as the substance and environment in which acute leukocytic
inflammatory cells muster to defend the host.
This zone varies with the degree to which topical care and hygiene
have controlled desiccation, injury, bioburden, etc. With scrupulously good care it can become
rather negligible (and the opposite with no care). There is also platelet aggregation here,
and this is the zone in which platelet-derived and other transformative
cytokines convert blood-borne monocytes into tissue macrophages. Zone 2 – GAG and
angio-attraction layer: This is the upper part of the aminoglycan
layer, at the boundary of the topmost plasma protein layer. Cell density is relatively sparse, and
there are no connective proteins here whatsoever. There are still neutrophils here (acute
inflammation), but not nearly in the numbers as above. There are three distinctive key elements at
this level. (1) The “space” is all
glycosaminoglycans, made by inflammatory and stromal cells, serving as the
“ether” in which the other cells operate until they can make an actual
fibrous matrix. (2) Large mononuclear
cells can be found here, monocytes and macrophages, making the proliferative
cytokines which induce the local repair cells. (3) “Planktonic” or migratory angiocytes,
generally individualized and spindle shaped as they stream from established
vessels below toward the source of chemotactic stimulation above. They can also be seen starting to
reorganize, becoming ovoid again as they start to reassemble with others of
their kind. Zone 3 – GAG and
angio-organization layer: This is the deeper part of the aminoglycan
layer. Neutrophils can still be found
here, but mostly in scant numbers, representing inflammatory chemoattraction
and migration rather than any type of injury or assault. Connective proteins are still missing. The distinctive feature of this level are
the angiogenic cords, reflecting angio-organization and the reformation of
tubular blood vessels. The angioid
cells and their cohesion are still a bit loose and immature, the cells still
big and unsettled, but they have found their positions, conducting channels
are open, and erythrocytes are present in the lumens. The new vessels have a distinctive look of
long radial or vertical cords traversing the gag layer. This establishes the environment in which
other cells can appear and do their functions. Zone 4 – GAG-connective histio-attraction layer: This is the layer where
collagen and matrigenesis begin.
Aminoglycans and new vessels are still the dominant anatomy, but young
fibroblasts are now present, and they are beginning to make young
collagen. Neutrophils are completely
absent, meaning that all afferent wound events are gone and the focus is
exclusively on repair. Vessels are better organized, some mature, and some
are of greater diameter, indicating that they are now supplying a downstream
angiosome of vessels organizing in the upper layers. Histioblasts, i.e. progenitor fibroblasts
have been stimulated into activity from mature vessels underneath, and young
fibroblasts have appeared and are proliferating. They appear as small round uniform cells
scattered between the nurturing angiogenic cords and young vessels. They are migratory, and they have little or
no organization, yet to be trapped in the collagen they will make. However, they are starting to make young
fibrillar collagen, which at this point is relatively non-descript – amorphous,
pasty, and homogeneous. Zone 5 – Amorphous collagen histio-organization layer: In zone 4, young
fibroblasts appeared. In this layer, young
fibroblasts are getting denser and making denser collagen, enough that
connective proteins, while still young and amorphous, have nonetheless become
the dominant substance of the medium.
There are no neutrophils.
Vessels are mature, some of greater diameter and mural thickness
reflecting a mature hemo-conducting network.
Fibroblasts have become very numerous and dense. They are no longer migratory, and some are
becoming trapped, but they are still more young and round rather than mature
and flattened. Young collagen fills
most of the space, the aminoglycans having been almost completely
displaced. The collagen matrix is
starting to look more fibrous, but it is still immature. This can be considered young scar, but the
overall architecture is still more wound than scar. Zone 6 – Fibrous collagen layer: Collagen has become not
only dense, but highly fibrous and well organized into lamellae or sheaf-like
bundles. Fibroblasts are mature,
trapped and flattened, settling in for a lifetime of collagen turnover and
remodeling. Arteries, veins, and
lymphatics can all be discriminated.
This layer can be considered real scar, and the end of the mesenchymal
component of wound healing. |
|||
|
|
|
||
|
This and the next two slides will show more details about each
of the wound events, strata, and physical findings. On the left edge is a vertical wound
image. It seems to be split into
almost exact thirds, each area with its own distinctive architecture. The “exact thirds” split is just an
artifact of how the photograph was cropped, but it does clearly illustrate
the progressive development of wound anatomy as the physiological events
evolve. The upper third has zones 1,
2, & 3 – inflammation, angio-attraction, and angio-organization – zones
made of plasma then aminoglycans without connective matrix, The middle third is the area of young
fibroplasia, zones 4 & 5, the histio-attraction and histio-organization
layers where fibrous matrix is being made.
The bottom third is zone 6, the fibrous collagen layer, the formation
of a scar and the conclusion of the mesenchymal wound events. The rest of the illustrations and text on
these 3 slides will look at the wound by its timewise physiological events. 0 - Active injury & inflammation. Inflammation is the initial response to
injury, to contain damage, clear debris, and prepare for repair. It is also the trigger for repair. Inflammation and repair are integrated
sequential processes. Left: a normal wound surface. Proteinaceous plasma exudates are the
medium, the only environment that exists at this level. What can live and function there are those
cells which normally live in plasma – leukocytes. Neutrophils are there in great numbers
because they are chemotactically attracted by inflammatory signals. Other leukocytes arrive in the wound more
or less in proportion to their concentrations in the blood, monocytes being
especially important as the keystone or bridge between afferent
(inflammation) and efferent (repair) wound events. Center: a close up view of normal wound neutrophils
in the upper plasma protein layer of the wound. Their presence indicates active
inflammation, disease, or injury of one sort or another. The greater the activity of disease or
injury, then the greater the neutrophils at this level and the greater the
degree of acute inflammation, and the less likely that the wound can
transition into the repair phase. Right: an injured leg, inflamed and not
healing. This was the result of a
superficial laceration in a healthy person.
Progressive dermatitis, panniculitis, and ulceration were a consequence
of inept care with injurious topical chemicals. Even when sustained injury does not result
in progressive ulceration, it will keep repair suppressed. 1 - Inflammation subsides.
Because sustained acute inflammation suppresses repair, it must subside
for repair to proceed. Left &
center: healthy wound surfaces
well cared for. These show the top
stratum, the plasma protein layer.
Under the influence of basic hygienic wound care (regular bathing,
silver based dressings, edema control), both of these specimens are nearly
devoid of neutrophils, stippled basophilia, nor any other evidence of
leukocyte activity and acute inflammation.
The cells that are present in the
upper plasma layer are all large and migratory – monocytes, macrophages, and some
arriving angioid cells. The subsidence
of inflammation means release from inhibitors that suppress reparative
events. Assuming these wounds are
otherwise healthy, they can now start healing. Right: the same clinical case after 2 weeks of
care. Acute injury and inflammation
are gone, and the wound is now healing. 2 - Macrophages, eschar separation. Macrophages are monocytes transformed by
inflammation. They have multiple
functions in the wound. Their afferent
function is as phagocytes that clean up the debris and damage of the acute
injury and subsequent acute inflammation.
Left: mononuclear cells are
distinctive in the topmost plasma protein inflammatory layer, appearing as
typical “compact” (blood borne morphology) monocytes, or in transition as
they accumulate cytoplasm and nucleoplasm, or as fully matured
macrophages. Center: this image shows the cleavage plane between
necrotic eschar (above) and viable tissue (below). The cleavage plane represents tissue lysis
and processing by neutrophils and macrophages. Right: eschar separation seen clinically. This is a pelvic pressure ulcer several
weeks after the pressure exposure and necrosis. The separation will continue until
complete, all necrosis eliminated, leaving behind healing wound surfaces. |
|||
|
|
|
||
|
3 - Aminoglycan ground substance. In normal tissues, the glycosaminoglycan
(gag) ground substance is the interstitial “gel” that fills the space between
cells and connective protein matrix.
In normal embryogenesis, it appears as the preliminary medium for
histogenetic cells, the “ether” that they require to migrate and organize
until connective proteins appear to stabilize their architecture. After injury to fetal tissues, “healing” is
simply the production of new gag ground substance, and then the restorative
generation of new cells and connective matrix as occurred during primary
histogenesis. The post-inflammatory
wound healing “program” does not become active until near-term or
peri-parturition. (The possibility of
suppressing “wound healing” and restarting embryonic histogenesis is one of
the “holy grails” of wound healing arts and science). However, the gag’s have a crucial role even
in normal wound healing. The
aminoglycan ground substance is required for the scar or stroma to form, because
angiocytes and fibroblasts and the vascular and connective structures they
form are just new tissue that needs the gag’s as a host medium. Their presence is critically important in
the earliest phases of repair, because angiocytes, the first of the repair
cells to appear, must have an aminoglycan medium or environment in which to
migrate and assemble. Left: a view just below the topmost plasma
protein layer. Pink plasma “puddles”
are present, but most of the “space” here is pale or unstained aminoglycans. Low cell density is typical, with some
neutrophils, monocyte-macrophages, and the “advance guard” of arriving
angiocytes. There are no connective
proteins here, and cell-to-cell organization and assembly which are just
beginning are still loose and amorphous.
The aminoglycans are made by various cells, but mostly by the arriving
angiocytes themselves. Center: an alcian
blue stain. H&E histology allows
the location of the glycosaminoglycans to be inferred, but it does not
directly stain the gag’s. Alcian blue
is the opposite, staining only the tissue gag’s (it stains the carboxylated
and sulfated aminoglycans of the “ground substance” such as chondroitin,
hyaluronan, dermatan, keratan; a red counter stain is used to reveal cells). The plasma protein top layer does not
stain, nor do the collagen layers below.
In between, the dense blue stain is the aminoglycan zone. It has two strata. The upper half is the angio-attraction layer
where, in response to macrophage stimulation, individual angiocytes are
streaming and arriving and starting to reassemble into new vascular
structures (the scattered lucencies).
The lower half is the angio-organization stratum where angiocyte and
vascular reassembly is complete, showing the vertical architecture of the
angiogenic cords and young vessels. Right: normal wound mucus. This is a proper part of any healing wound,
and absence of this layer or these chemicals is associated with weak
angiogenesis and impaired healing. The first three events of wound healing
– subsidence of inflammation, macrophages, and ground substance – are the
afferent wound events, the preparatory or pre-matrix phase, when things are
cleaned up and readied for the formation of new stroma. The next four phases – angiogenesis,
fibroplasia, contraction, epithelialization – are the efferent wound events,
the repair activities. The purpose of
wound healing is simply to repair the basic fibrous stroma, consisting of an
architectural superstructure (connective protein matrix) and a logistical
supply network (blood vessels). This
is effected by two mesenchymal cells – angiocytes and fibroblasts. New repair cells are derived from existing
local stem-regenerative-pluripotent cells, mainly existing vascular cells in
adjacent blood vessels (there may also be a contribution from circulating marrow
or other remotely derived stem cells).
Keep in mind that the order of appearance and inter-operative dynamics
of these cells and their derived structures is different for wound healing as
compared to normal embryonic histogenesis.
Embryogenesis makes normal stroma.
Wound healing makes scar, a dense disordered stroma which must
eventually remodel back to a normal stromal histology (maturation). 4 – Angiogenesis.
The efferent function of the macrophage is to make cytokines or
peptide growth factors which initiate and attract repair cells. In normal wound healing, angiocytes have
precedence. They must appear first and
make new vessels and re-establish circulation (logistical supply) before
fibroblasts can appear and function to make the connective matrix. Because they are operating where there is
no structural matrix, they make their own medium, the aminoglycans, where
they can migrate and maneuver and reassemble into vascular conduits. (This is different than normal
embryogenesis, where local parenchymal cells appear first, and attract new
vessels only as required to maintain proper vascular density and circulation
in the developing tissue or organ.)
New angiocytes are derived primarily from existing nearby blood
vessels.
Transformative-mitogenic-proliferative angiogenic growth factors from
wound macrophages diffuse outward, and where they impinge on surrounding
vessels, angiocytes get activated.
Cytoplasm and nucleoplasm increase, cells mitose, and they peel off of
the parent vessel and start migrating toward the source of the stimulus. As they reach the target zone, they
coalesce or reassemble into angiogenic cords which, as they become integrated
back into the established vascular network, begin to conduct blood flow. Only once this has occurred can fibroblasts
then appear and function to make the connective matrix. Left: the upper half of the aminoglycan zone, the
angio-attraction stratum, where mononuclear
cells (monocyte-macrophage) are signaling angioid cells from vessels below. Dis-associated individual angiocytes (long
spindle cells) are streaming toward the source
of chemotactic stimulation and reorganizing piecemeal into vascular
structures (clusters and cords). These
are events which are taking place just below the plasma protein inflammatory
layer, roughly 3-5 days after a single-event injury with healthy wound
healing. Center: the lower half of the aminoglycan zone, the
angio-organization stratum, where angiocytes have reorganized into
structurally competent vessels connected to the general circulation, with
open lumens and conducting blood flow.
The vertical or fan shaped arrangement of the new vessels is
characteristic. These are still
immature vessels, and they will remain young and dynamic for some time yet,
because not only do they need time to mature, but they are now themselves the
interceptors of macrophage cytokines and the source of new angiocytes for
ongoing afferent wound events taking place in the strata above. These events are roughly at 4-7 days in the
healthy normal wound. Right: typical “granulation tissue”, i.e. the
clinical appearance of the aminoglycan and angio-organization layers. 5 – Fibroplasia.
Once new vessels have restored a good environment, fibroblasts can now
proliferate and create the fibrous structural matrix which gives stability
and mechanical competence to the regenerating stroma. Left: young new fibrous cells and connective
matrix (staining pink with h&e) among re-established vessels. This is zone 5, the histio-organization
layer, where aminoglycans are no longer the most voluminous substance in the
composite material. These events are
roughly at 5-10 days in the healthy normal wound. Center: advanced fibrous collagen production has
occurred, giving the new stroma mechanical stability. This is zone 6, the fibrous collagen layer,
the young scar. Right: in a healthy wound, fibrosis can be
inferred by the mechanical characteristics of the tissues, but it usually is
not seen because “granulation tissue” and the upper strata hide what is
underneath. However, in a wound with
atrophic upper layers (which is pathological and not likely to heal), slowly
developing fibrosis is easily seen. |
|||
|
|
|
||
|
6 – Contraction.
As fibroplasia progresses and scar forms, something amazing also
happens – scar and wound contraction. Some
fibroblasts have actin and myosin and other markers of muscle
differentiation. Unlike in true
muscle, these contractile proteins do not become highly ordered, periodic,
and synched between cells, “crystalline” if you will, nor do they require
myoneural action potentials to trigger.
However, they are present in certain individual fibroblasts, aka
“myofibroblasts”, for the same purpose as any muscle – to contract. Their effect is to diminish the surface
area of the wound, cranking or ratcheting the surrounding native tissues back
together, and thereby also minimizing the proliferative and migratory load on
surrounding epithelium which has the final responsibility for “closure”. Left: new scar, with typical features responsible
for its mechanical properties. The stratification, condensed organization, and dense packing of the
collagen fibers is obvious. The scar
bundles are thick, and different bundles crisscross in different directions,
resulting in loss of elasticity and compliance. Center: dense, highly cellular scar from a “genu”
of contraction subjacent to an infolding wound margin. Myofibroblasts cannot be discriminated from
regular fibroblasts by conventional light microscopy color stains (h&e,
trichrome, etc.) – rather, e-m and immunos are required. However, gross scar architecture is
different in areas of contraction, straighter
and
more orientated and aligned with the direction of contraction – no surprise
since there must be a dominant force vector and loss of isotropy if the scar
is to be able to deform in a given direction. This is the “rubber band” of myofibroblast
activity and wound and scar contraction.
Right: a wound actively
closing by contraction. This wound is
substantially smaller in area compared to its initial size, as evidenced here
by the narrow and now-epithelialized vertices, and also by the curling inward
and downward of the skin margins toward the wound base. Fibroblasts and myofibroblasts react to
tension in predictable ways (see slide 27), and as a self-organizing system
(all about this concept in Part 3), the cells will automatically level
tensile loads across the surface, meaning a reduction in algebraic order or
net geometry of the curvature of the perimeter and surface, i.e. getting
progressively smoother and rounder as they get smaller. 7 – Epithelialization.
A wound is nominally closed when epithelium is continuous, and the mesenchyme
is therefore fully sequestered from ambient world. Complete
epithelialization is the nominal endpoint of wound healing for the sake of
practical everyday wound management.
Epithelium migrates only over other healthy wound module components. Left: epidermis at edge of a healthy wound. What were normal basal
cells and acanthocytes have become primitive and migratory, streaming outward
toward a wound margin that has a suitable wound module underneath, especially
well-formed superficial capillaries.
Migrating epithelium bears little resemblance to its mature form, but
the cells maintain contact with each other as they spread superficially and
tangentially in an elongated flattened form.
Center: advancing
epithelium cleaving eschar. Epithelium
needs a healthy stroma to migrate on. either a restorative stroma (wound
module) or native stroma if the tissues are relatively uninjured. This view is comparable to the eschar
cleavage plane seen on slide 8, but here, epithelium is directly finding the
boundary itself in an area where acute inflammation and leukocyte-macrophage
events have not yet fully developed. Right: clinical view of epithelial ingrowth. Active
epithelial ingrowth is occurring from all wound margins, covering granulation
tissue that has already formed. This
process will continue until its growth is inhibited by contact with itself,
and the wound is then closed. 8 – Maturation.
Once epithelialized, wounds mature.
This is a slow involution or
remodeling wherein the young scar’s dense over-abundant excess of collagen,
fibroblasts, and new blood vessels is gradually removed, and the scar
progressively returns to the mechanical and histological characteristics of
the native stroma. Stroma can of course
have various forms – dense fascias, areolar fascias, musculoskeletal
fascias-ligaments-tendons, and dermis or various tunicas. Whatever is the correct architecture for
the given location, host structure, or intent, the generic young
fibro-vascular material, the scar, will respond to local mechanical forces and
biological effects to slowly regain the gross and histological morphology of
its host structure. Left: mature scar returning to dermis or fascia. As scar
becomes progressively mature over a period of months to years, fibrocyte and
collagen density decrease, and collagen bundles become wavy and springy, with
tangential spaces or planes opening between them. Vessel morphology returns to normal, and
the number of vessels diminishes back to normal vascular density. This is all apparent in the fully matured
scar depicted. The herringbone pattern
attests to a final collagen configuration that is once again compliant and
mobile. Vessels are sparse, and
fibrocyte density is at a normal minimum.
While not looking exactly like normal dermis or musculotendinous
fascias, it looks very similar. Center: epidermis maturing, and forming a
lamina propria (the papillary dermis).
Regenerated epithelium also matures, slowly developing all of the
attributes and functions of its native parenchymal form. For epidermis, this means the restoration
of papillae, basement membrane, and the various functional and anatomical
strata. It also means the formation of
a lamina propria (papillary dermis), a service layer on top of the primary
(reticular) dermis to supply the high metabolic requirements and parenchymal functions
of the epithelium. Right: the same leg as 0 & 1 (slide 8), healed
and mature. |
|||
|
|
|
||
|
And now, for something entirely redundant. But necessary. As a prelude to everything else that is to
follow in these 3 lectures, the details of wound healing must be distilled to
a few quintessential concepts. First,
the response to injury is an integrated series of linked events. The first event is that something recognizes
injury. Thrombosis is usually credited
with this accolade, which while inherently true is also an incomplete
explanation. Thrombosis does indeed recognize
many injuries, but other mechanisms such as allergy and immunity also recognize
the primary assault on the body (discussed in detail in Parts 2 &
3). Regardless of how injury is recognized,
the response is inflammation.
Inflammation is the body’s generic protective response, mediated by
blood borne leukocytes. As part of
their response to injury, they initiate the afferent events of wound healing. These are mediated by macrophages, which
are simply blood borne monocytes converted to their tissue phenotype by platelet
derived transformative cytokines.
Macrophages clean up the injury, paving the way for repair, then they
initiate repair by issuing their own set of transformative cytokines which
stimulate local repair cells. The efferent events of wound repair are the restoration of the
mesenchymal stroma, then sequestration of the mesenchyme from the ambient
world by the restoration of epithelium.
Mesenchymal repair is due to two – and only two – cells, angiocytes
and fibroblasts. Keep in mind the
essential biology of all of this.
Multicellular life, with specialization and division of labor among
cells (and the complexity and adaptability that they confer), is wholly
contingent on just a few crucial elements.
The two categorical necessities are (1) an architectural structural
framework where differentiated parenchymal cells can be housed, and (2) a
logistical distribution network that allows parenchymal cells to deliver and
receive items to and from each other.
This generic “framing and utilities” is the generalized stroma, and it
exists everywhere in the body in one form or another to support epithelia and
parenchyma. It is composed of just 2
cells, angiocytes and fibroblasts, and the fibrous and vascular structures
that they make. Wound healing is
nothing more than this stroma restoring itself, enough to re-establish the
structural competence of the injured area and allow parenchymal cells to
replenish themselves. To reiterate, the overall response to injury, inflammation then
wound healing, occurs via two general populations of cells – acute
inflammation and wound module. Acute
inflammation can be subdivided into two groups, the thrombosis or
injury-recognition events (plasma, platelets, allergy-immunity, etc.) and the
acute inflammation events (leukocytes, especially neutrophils and
monocytes). Wound module can also be
subdivided into two groups, mesenchymal events (angiocytes and fibroblasts)
and epithelial-parenchymal events. Repair
per se is contingent on 4 specific cells:
(1) blood borne inflammatory monocyte-macrophages are the instigating
link between inflammation and repair, (2 & 3) histio-fibroblasts &
vascular angiocytes are the mesenchymal cells which restore the generic stroma,
and (4) keratinocytes or other epithelia are the parenchymal cells which
“hang” on the stroma, sequestering the mesenchyme and restoring functional
competence to the injured area. |
|||
|
|
|
||
|
This and the next slide are here to reiterate that the
mesenchymal component of repair is based on 2 cell types, angiocytes &
fibroblasts, this slide focused on angiocytes and the vascular structure they
make. Left:
a view of the aminoglycan angio-attraction layer. Spindle shaped angiocytes are streaming
from vessels underneath toward macrophages in and above this layer (the other
round cells are mainly neutrophils, normal at this level). Center: a close up view near the
top of the wound, at the plasma protein and aminoglycan boundary, having
mononuclear (monocyte-macrophage) and angioid cells. The cell cluster is a vessel reassembling
from individual angiocytes. Right:
a wide view a bit lower, in the angio-organization layer. Angiocytes have formed long vertical cords
and conducting vessels, reaching from mature vessels underneath toward the
chemotactic stimuli above. Once these
vessels and blood flow are established, the fibrous component of repair can
start to develop. |
|||
|
|
|
||
|
This slide emphasizes the vital role of fibroblasts in the
repair process. They appear after
vessels have established an environment in which other cells
can proliferate. As with angiocytes,
they are sourced from pluripotent mesenchymal cells in the vascular loci
below. Fibroblasts make the connective
matrix, and myofibroblasts contract the wound. Left upper: early fibroblasts are interspersed among
organized vessels in the histio-attraction stratum. They are numerous and small, of
non-specific shape. The medium is
still largely aminoglycans, but thin strands of eosinophilic young collagen
are starting to appear. Left middle: a view a bit deeper. There are vessels at bottom and upper
right, and between them young fibroblasts are larger and denser, consistent
with proteogenic activity, and more of the space is occupied by pale pink
collagen. Left lower: a view yet deeper in the
histio-organization layer. Young
fibroblasts remain dense, and the space is now almost completely filled by
young disorganized collagen. The cells
are, in general, less round, more spindled, and starting to take on some
organization in the form of stratification or lamellations. Right middle: the next view, going yet deeper into the
wound showing young scar. The randomly
arranged young fibroblasts are starting to become flatter and layered, trapped
and stratified between maturing bundles of wavy pink collagen. Right lower: at yet a deeper layer, the stratification,
organization, and packing of the scar is obvious. The scar bundles are thick, and they
criss-cross in different directions. Right upper: a view from the wound margin subjacent to
an infolding skin edge. This is the
dense directional scar associated with myofibroblast activity and wound and
scar contraction. |
|||
|
|
|
||
|
This slide reiterates that the reason angiocytes and fibroblasts
proliferate is to restore generic stroma, the basic framework of connective
matrix and blood vessels that supports parenchymal and epithelial and all
other cells. Left:
an example of “granulation tissue” dense with vessels. This is from a wound chamber implanted
explicitly to raise a crop of wound activated cells (see slide 27). This specimen has almost exclusively
angiocytes and erythrocytes, with no inflammatory cells and only a few young
fibroblasts or histioblasts.
Fibroplasia will follow, but without the vessels there first, nothing
else can grow and be productive. Center:
the middle to lower strata of the wound, where newly organized
angiocytes and vessels mix with young fibroblasts and the connective matrix
they are making. Right:
the gross appearance of active wound fibroplasia. In this abdominal
wound, the aminoglycan-angiogenic strata are atrophic and thin, allowing the
deeper layer of fibrosis to be seen. |
|||
|
|
|
||
|
This slide reiterates that the wound and scar is nothing more
than the restoration of the stroma, and that although it appears first as a
generic “scar”, that with time it will mature back to a connective
architecture typical of its host tissue. Left:
a set of scars after several operations. Some are young, thick and stiff from excess
connective matrix, discolored from excess vascularity and hyperemia. Some are old and mature, being pale and
flat, soft and compliant as time has resolved the excesses of the young scar. Right upper: fibroblasts, collagen, and new blood
vessels are seen at the peak of proliferative repair. Right second: a skin scar weeks after full epithelialization. Vascular and fibroblast density have
lessened from their peak in the top image. Right third: a maturing scar (months), with wavy collagen
bundles, spaces opening between them, and decreased cell and vessel density. Right lower: a matured scar (years) with open compliant
collagen bundles and sparse vessels and fibrocytes, much closer to normal
dermis or musculotendinous fascias rather than scar. |
|||
|
|
|
||
|
We saw these photos on slide 3.
The normal skin is static, without any activity or reactivity absent
any injury or provocation. Wound
healing is a reserve system, on standby until the proper trigger occurs. Once triggered, it runs its course until
the wound is healed. The conventional
bioscience aspects of that process were reviewed on the preceding
slides. Once the wound-stroma-tissue
is restored to normal, wound healing ceases.
This brings us back to the initial inquiries of this talk: (1) How does the wound healing process know
when to start, how to execute its business, and know when to stop? (2) Why do some wounds fail to heal, even
when components of the healing process appear to be active? The answers begin by understanding that the wound is a
non-linear control system. The answer
to question 1 is in the normal physiological operations of this control
system. The answer to question 2 is in
the pathological or disordered behaviors of this control system. The basic concepts of non-linearity and
control were alluded to on slide 3.
Here we will start to explain why normal wound healing is a control
system, first looking at some general principles of closed loop control. As explained on slide 3, biological systems must remain within
certain bounds if they are to function properly. The same is true for nearly any system,
natural or engineered. Many systems
have fairly precise or narrow-range target values. Those target values are the system
reference. Closed loop control systems
have the “machinery” necessary to sense their own actual state, compare that
to the system reference, then react to correct any differences between the actual
and the target values. Closed loop
control systems generally have the following components. Reference: This is
the target or value that the system is meant to be at, that the control loop
works to maintain. Nodes: These are combinatorial
points that yield values. The main
node, where the system state is compared to the system reference, is usually
indicated as a “summation point”, a simple comparator where one value is
subtracted from (or its inverted value added to) the other. Error signal: This is
the output of the primary summation point, indicating variances of the system
away from the reference value, Controller: This is
the component that is directly influenced by the error signal, the first part
of the mechanism needed to implement corrections. It is like the executive or general that
receives all of the intelligence from the field then must issue orders to the
troops to take some action. Load: This is the element
acted on by the controller, the actual productive machinery which will change
the system state and try to correct the system error. These are the employees or troops who,
commanded by the controller, do the real work of creating the company output. Output: This is the productive
output of the controlled load, that which modifies the state of the system,
bringing the system back toward the system reference. Feedback: Some method
is needed to measure the current state of the system and report that back to
the comparator node. Forces: Extrinsic to the control
loop itself, these are applied stresses or perturbations which alter the
state of the system, forcing a response from the control loop to try to
restore the reference state. They can
sum into the loop at nodes which, depending on the specific nature of the
system, can be located almost anywhere on the loop. Note that there is no start or stop, no on-off switch. As long as the system as a whole is “on”
and active, the control loop is free running, always striving to restore the
system to its reference value. If the
reference value is reached, then the output of the main summation-comparator
node is zero, and there is no force driving the loop. As soon as there is any intrinsic decay or
drift in the state of the system, or any perturbation due to extrinsic
stresses, then the compared value is no longer zero, and an error signal is
generated and the loop is driven. The feedback and closed loop are by definition “non-linear”. In conventional mathematical terms,
“linearity” can mean several things depending on context. As applied to dynamics. linearity means a
functional relationship between two variables (a function being the classical
algebra-calculus-topology definition of a “one-to-one and onto” relationship,
embodied in an equation of the form y = f(x), where there is only one
allowable value of the dependent variable for any value of the independent
variable). For tangible systems in the
real world, dependent-versus-independent functional relationships are wildly
diverse, They can be as simple as a
simple scalar of the form y = Ax (straight line “linear” in another
sense), or higher order polynomials or angular-trigonometric functions or a
zillion other things. The system state
(dependent variable) may be a function of time or position or whatever
(independent variable), but all have that quintessential definition that the
system output value is uniquely determined by the value of the input. In contrast, non-linear systems are those
where the system is a “function” of itself.
Rather than the value of the system being mapped against time or
position or whatever, its output value is determined by its current
value. Its value now is the
input (comparable to the independent variable), and its value next is
the result (comparable to the dependent variable), but there is only one
variable involved, the system parameter that you are observing. In a linear system, you can arbitrarily
pick a value for the input variable, and then calculate precisely the value
of the output variable. In a
non-linear system, you can pick an arbitrary initial value of the system, but
then you must sequentially recalculate the next value of x versus the
current value of x. Each time
you recalculate, the latest output becomes the next input, each such
turnaround known as an iteration.
If you want to know what the value of x is after 500
iterations, you cannot simply plug “500” into time t in an equation of
the form x = f(t); instead you
must run 500 iterations to finally get to the value you seek. We cannot write x = f(x), because
that is the notation for a function, and non-linearity is not “functional”,
so instead we write x § f(x) to denote the
self-dependent, recursive, iterative nature of the system. As will be explained in detail in Part 3, there are many systems
in mathematics, engineering, and the natural world that do not have a
functional relationship, i.e. many non-linear systems. Compared to the tools of algebra and
calculus, the mathematical tools required to work with such systems are of
more recent advent, and not so easy to work with, so they get little
attention in basic math and science education. Nonetheless, this is the stuff of complex
systems, the stuff of the real world.
Control systems are all of this form.
When a controlled system feeds back, its own state becomes the next
input into the system, Non-linearity
is inherent in feedback. Closed loop
control means non-linearity. The state
of the system does not have a functional linear relationship to time,
distance, energy, momentum, temperature, nor anything else. The state of the system in the next
iteration (the output) depends only on its current value (the input) as transformed
by the operational functions of the system (the f in x § f(x) ) that govern
the physics or chemistry or other dependencies between individual components
of the system. For non-linear systems
in general, their output or behavior can have several generic forms, as
described on slide 23. For closed-loop
reference-driven controlled systems, the general idea is for the system to
stabilize or converge on a target value. |
|||
|
|
|
||
|
We have already established that biological systems must remain
within bounds if they are to function properly. That is why there is a need for
control. Each system and subsystem
needs a mechanism to pull itself back in bounds when it decays, drifts, or is
pushed too high or too low out of its effective operating range. Control lets a system hold itself to the
intended reference, output, state, or attractor. Key to control is feedback, the ability to
sense the system state or output, which then drives an error correcting
mechanism. To understand why control is so important, first consider an
arrow. The archer aims, then
releases. He hopes his aim was
good. However, once the arrow leaves
the bow, nothing can be done about it if the aim is off. There is nothing on the arrow itself that
can assess nor correct its own trajectory.
Even if the aim was perfect, a gust of wind can throw it off. Without feedback, this system is open
loop. Without feedback and a response
mechanism, there is no control. Open
loop systems must be calibrated (aimed), and if anything upsets that calibration,
the system misses its mark with no recourse.
In this open loop arrow system, we can diagram the “machine”. The same terminology applies as for any
generic control system: the target is
the system reference, the controller is the archer, the arrow is the
controlled load, and the output is the trajectory and impact point. But note that there are crucial differences
– there is no feedback loop nor error signal.
In lieu of feedback (which will continuously update and correct the
system), there is instead just a calibration (a one-time up front event). Many man-made machines are open loop, because they can be made
stable, without drift or decay, and they can be isolated so as not to be
affected by outside forces. However,
biological systems all swim in a sea of stochastic variability, predatory
perturbation, and “things that go bump in the night”, stresses that by intent
or happenstance will strain systems away from their operating ranges. In the kingdom of life, open loop
calibrated systems are of marginal value.
Biosystems, at small molecular scales and at massive population scales
and at every scale in between, all need ways to regulate or correct their
state if the system is to function and stay alive. Contrast the arrow with an airplane. The airplane is steered, not aimed. This is because there is feedback in the
system – the pilot and his instruments and flight controls repetitively assess
and correct the plane’s heading. Just
as with the arrow, the destination is the system reference, the pilot is the
controller, the aircraft and more specifically its flight control surfaces
are the controlled load, and the output is the aircraft’s heading. In both systems, external forces such as
wind sum into the loop, which for the arrow can mean an irreparable loss of
calibration. For the airplane though,
perturbation of the system state (the output or heading) can be sensed and
fed back via the navigation instruments.
If there are variances between heading and destination, then an error
signal is generated (the gauges on the instrument panel), and the controller
(the pilot) can actuate changes in the load (the flight controls). Feedback and closed loop control assure
that system reaches its target, even when perturbed. |
|||
|
|
|
||
|
Are you a conventional bioscientist and finding this discussion
about physics and engineering a bit foreign?
Does the concept of “system” beyond rudiments like “cardiovascular
system” and “musculoskeletal system” seem arcane or irrelevant? Do feedback and control seem to you like
concepts on the fringe of biology? If
so, you are in good company, because of the quirks of science and science
education over the past 150 years. Twentieth
century biological science, systematology, and academics are colored by the discoveries
of the latter 19th century, when chemistry, organic chemistry, and a
scientific pharmaceutical industry turned everyone’s attention to
biochemistry and the chemical basis of metabolism and disease. Remember when organic chemistry was the
‘acid test” of premedical education?
Physics, as a rigorous disciplined predicate and also as a sister
subject to the study of physiology and pathology has been given little
emphasis during this era. The legacy of this attitude is that everyone assumes that every
dynamic in the body is comparable to a chemical reaction, and that everyone
is looking for the chemical basis of what goes wrong. A simple example for wounds concerns
nutrition. Those who know nothing
about wounds all think that all you have to do is stuff the patient with lots
of carbs and protein, and that a wound will then heal. As foolish and ignorant as that concept is,
it has an historical basis. Twentieth
century physiology was informed largely by chemistry, where simple chemical
reactions have simple reaction kinetics.
Consider a basic chemical equation such as [A] + [B] D [C]. In a test tube, you can pour more substrate
on the left side of the equation, and more product comes out on the right
side. Therefore, so the impaired
thinking goes, just pour more chicken fried steak and coconut cream pie into
your patient, and all things metabolic will speed up, and the wound will
churn out collagen faster than you can give it a haircut, and your wounds
will then magically disappear. No, of course not. But
that is the legacy of 19th and 20th century science on
the mentality of physiology. This
approach to physiology was biased towards basic chemical dynamics,
biochemical dynamics (e.g. Michaelis-Menten enzyme-substrate conversion), and
the linear characterization of how any 2 chemicals react. For example, the Krebs and TCA cycles could
be mapped by looking at the basic kinetics of dual-species conversions, such
as citrate-aconitate or fumarate-malate.
However, understanding the integrated composite behavior of these
cycles and their many interconnects could only be reduced to those big highly
interconnected wall charts that overwhelmed you in medical school (see Part
3, slides 16 & 17). Trying to
understand-model-predict the overall integrated operations or states of
highly interconnected complex systems was beyond the analytical or
computational means of those times (n-body dynamics; see Part 3). Instead, biological research and doctrine
became anchored in the dependent-versus-independent method of experimental
biology based on simple linear interactions.
This mentality extended from the test tube to the clinic, where RCT’s
– randomized controlled trials – became the gold standard of exploring the
effects of a single agent or intervention on the net behavior of a
system. This approach to science has
substantial limitations, because it cannot ever elucidate the comprehensive
integrated behavior of complex n-element systems. Nonetheless, complex, non-linear, and
n-body systems were deprecated as “intractable”, and consequently not worthy
of study. Within the halls of
biomedical academia, there was, and largely still is, no room for
understanding nor teaching overall systems biology (but as discussed on slide
5, that is beginning to change). Much of what we would now consider systems dynamics was simply
rolled into the overarching idea of “homeostasis” as defined by Claude
Bernard and Walter Bradford Cannon.
This concept, that organisms or systems can maintain their “internal
milieu” in a stable desirable state, is achieved through various regulatory
mechanisms and dynamic equilibria or steady states between components of the
system. We recognize easily that this implies
“control”, that the “desirable states” are the system references, and that
the regulatory mechanisms are simply the various control blocks and signals
within a control loop. Note though
that “equilibrium” was an important part of the way homeostasis was
envisioned on the cutting edge of 19th – 20th century
biology, rather than recognizing that complex systems are more likely to have
stable but non-equilibrated chaotic attractors (see Part 3, slide 20). The confusion is understandable though,
because back then chemical equilibrium was an au courant concept of the
times, and non-linear dynamics was a fantasy world that few could even envision. The reality though is that non-linearity
and control are the core dynamics of all biological systems. However, there is at least one closed loop
feedback system that has been a classical part of medical education for a
long time - the hypothalamic-pituitary-endocrine system. When it is taught in medical school, it is
often portrayed as an interesting oddity that a feedback system even exists .
. . not true at all, but this makes it a convenient place to start to
illustrate control & non-linearity in biological systems. The endocrine control system has been abstracted here to a
uniform loop that actually regulates several different organs and system
references. Whether dealing with
thyroid, adrenal, gonads, or growth, the system reference is some manifestation
or measure of “normal metabolism”. The
feedback in the loop is the level of end-organ hormones (thyroid, adrenocorticoid,
gonadal, anabolic), either those hormones themselves or some metabolite of
them or some further downstream metabolite resulting from their biological
effects. The system comparator is the
hypothalamus, which through some black-box mechanism monitors the balance
between the reference value and the actual concentration of hormone or
metabolites. If there is a variance,
then the hypothalamus issues an error signal in the form of the releasing
hormones (trh, crh, gnrh, ghrh). This
signal drives a controller, the pituitary, which amplifies the signal and has
the “power” to drive the system load. It
drives the load via a control signal in the form of the stimulating hormones
(tsh, acth, fsh-lh, gh). The load
itself is the peripheral endocrine organs and other tissues – thyroid,
adrenal cortex, gonads, and liver & mesenchyme. The system output is the set of peripheral
endocrine hormones that regulate whole body metabolism and related functions
(thyroid hormone, cortisol, progestins, etc.). They or their effects are then transduced
and fed back to the system comparator. In human engineered systems, such as nuts-and-bolts machines and
electronic circuits, systems can be designed around a few well-defined
elements. In complex biological
systems, there can be dozens or hundreds of relevant elements, so control
diagrams such as this are abstractions that roll many elements together into
the main control blocks. There will
always be a variety of options for how these simplifications are drawn, and
how the many elements are represented by the few. For example, this loop could be drawn with the
pituitary and even the end-organs just being amplifiers on the hypothalamus
output, and some manifestation of general cell and substrate metabolism as
driven by the endocrine hormones could be listed as the load and output. Thus in modeling complex physiologies,
control loop abstractions, simplifications, and assignments are necessary,
meaning there is latitude in deciding exactly where and how to represent each
biological element on the circuit. For
the wound, which has substantially many contributing elements, abstractions
and simplifications are necessary, but the relative roles of the major
components of wound healing are clear enough that a standardized wound
control loop can easily be defined. |
|||
|
|
|
||
|
Like nearly every other system in physiology, the wound is a
regulated controlled “machine”. Like
everything else, the proliferative module of normal post-inflammatory wound
healing must be regulated if it is to remain within its operating range. It must be regulated so it knows when to
turn on, how much output to create, and when to cease. This control or regulation is a non-linear
process based on a closed feedback loop having all of the elements of any
well-controlled machine. The many anatomical
constituents and physiological events of the wound module all correlate with basic elements in the wound control system. Presented here is the Wound Main Control Loop. The next three slides will explain the
components in detail. The following is
a basic description. The system
reference is closed (epithelialized) normal tissue free of disease,
injury, and inflammation. How the body
recognizes this condition, i.e. what physiological or anatomical properties
constitute closure are biologically important, but they are not specifically
relevant to the basic control dynamics.
With regard to the loop and its dynamics, each of these control blocks
is the proverbial “black box”, made up internally of countless biological
parameters which somehow transform an input into an output, but looking to
its neighbors as just a single element with a well defined input or
output. The system reference is
continuously compared to the actual state of the wound, and any variance of
the wound away from “closed” generates an error signal. The system comparator and error detector
are the recognizers-sensors-transducers of injury, mainly leukocytes,
platelets, and related chemicals. These
are the initiators and mediators of acute inflammation, and what they do,
their activities and consequences – their output – is inflammation itself. Inflammation, its various chemical and
cellular effects, is the error signal, the way that the system
comparator tells the system controller what to do. The system controller is the
macrophage. The error signal boosts
macrophages and turns them on to do their afferent and efferent wound
functions. Their efferent function is
to issue pro-proliferative growth factors, the control signal which
will actuate and drive the system load.
The system controlled load is the set of cells which does the
actual work of rebuilding tissue and trying to correct the open wound
conditions which generated an error signal.
These cells are the angiocytes and fibroblasts which restore the
mesenchymal stroma, and the epithelial cells which close and sequester the
mesenchyme. These cells are the
load. What they create is the system
output, mainly ground substance, vessels, connective matrix, contraction,
and epithelialization, those things that are explicitly the new tissue which
reduces “open wound” and corrects the system error. Finally, the new state of the system must
be reported back to the system comparator.
The feedback is the residual open wound, with various “black
box” attributes of “openness” being transduced by the error detectors. Note the circle leading into the feedback
block. The system output has the
effect of diminishing the state of “open”, so that effect on the “open wound”
block is a negation, an inhibitory or inverting input, represented by the circle.
As described on slide 16 concerning general control systems, note
that there is no start or stop, no on-off switch. As long as there is an open wound, the
control loop is running, always striving to restore the system to its
reference value of closed. If the
reference value is reached, then the output of the main summation-comparator
node is zero, and there is no force driving the loop. On slide 16 we also stated that “as soon as
there is any intrinsic decay or drift in the state of the system, or any
perturbation due to extrinsic stresses, then the compared value is no longer
zero, and an error signal is generated and the loop is driven.” For the endocrine control loop, where the
reference value of hormone levels or metabolism is some positive value, then
intrinsic decay and drift are the variances which drive the system, and the
system will be continuously active and continuously driven from birth until
death. For the wound, extrinsic
perturbation is what drives the system, in the form of injury. When the wound settles back to “closed”,
there is no error, and the loop remains quiet. Injury instantaneously creates a condition
of “open wound” which enters the loop at the feedback block, immediately
generating an inflammation error signal, and the loop then runs autonomously
until the wound is closed. Knowing
when to start and stop is not a matter of knowledge or intelligence within
the system, it is just a matter of a well-tuned control loop operating as
designed. |
|||
|
|
|
||
|
This slide focuses on the front end of the Wound Main Control Loop,
the system reference, the error detector and comparator, and the inflammation
error signal that they create. System reference is a closed healthy tissue free of
injury and inflammation. This
condition means that the tissue is continuously epithelialized, the mesenchyme
therefore fully sequestered from the ambient world, and also that no acute
injury or defensive activities are present – just normal healthy tissue
carrying on normal metabolic or vegetative functions. The wound control loop works to restore these
conditions whenever the system state is disturbed, and the loop ceases when the
restoration is complete. Remember that
the state of “closed” is a complex set of biological parameters that must be
transduced and measured by the system comparator. The specific biological and biochemical
details of how that basal state is measured is a matter of conventional
biology, and knowing and understanding these “nuts and bolts” is a vital part
of understanding the whole picture of wound healing and wound pathology. However, from the dynamics and control
point of view, these various parameters are hidden inside the “black
box”. (The same is true for every
black box block on the loop.) Actually,
while these comments are true as a matter of general principles, the basal
state of “closed” is not transduced at all.
Rather, the basal closed state (normal tissue) is passive, and the error
detector simply ignores it and stays quiet.
The normal state is ignored because there are no active positive
tangible markers of openness. As
explained in the paragraph on the feedback element (slide 22), the condition
of “open” is what is actively transduced and sensed. The system reference therefore serves as a
ground or zero or bias on the comparator.
This comparator “knows” when the wound is open when positive markers
of openness are presented by the feedback block. The comparator “knows” when the wound is
closed when the markers of “open” vanish, making the now-healed wound equal
to normal tissue which likewise has no markers of “open”. (The photos illustrate this
fundamental restoration, from open to closed.) Error detector & system comparator is based on
blood borne elements. “Blood borne” includes
solid and cellular agents (leukocytes and platelets), soluble agents (plasma
protein coagulation system and acute phase paraproteinemias), and
miscellaneous blood and vascular parameters such as vascular stasis,
rheological effects, micro-angio-alterations, and any other acute state of
the blood that triggers some sort of protective reaction. These elements recognize and react to conditions
of injury or epithelial loss, thereby sensing the positive markers of
“openness” or “wound”. How it is that
plasma, platelets, and leukocytes are activated by injury is basic bioscience
knowledge and need not be reviewed in detail here. What is important from a dynamics and
control point of view is that the state of injury-wound-openness is
recognized and transduced by elements within the comparator block which then
generates an output signal based on those inputs. The internal workings of the black box are
not relevant as long as the “transfer function” or mapping from input to
output represents a consistent physiology or set of rules. The error detector and system comparator is
the keystone element in the control loop.
It has two inputs, the state of the system (wound or openness) and the
system reference (normal tissue and closed).
An error signal (inflammation) is generated when there is a variance
between the inputs. As mentioned in
the last paragraph, the state of “normal” is a passive input, i.e. normal
tissues do not excite or activate the error detectors
(plasma-platelets-leukocytes). The
state of “wound” is an active input due to tangible chemistry that is not
present in the “normal” state. Note
the “plus +” and “minus -” signs on the comparator inputs. The minus indicates that this is an
inverting or negating input, and the plus indicates a positive or summating
input. “Negative” is subtracted from
the “positive” to get the output value of the node. When the absolute value of “open wound”
exceeds the absolute value of “system reference”, then the output is positive
and the loop is driven. See slide 22
for specifics of how the system recognizes the state of openness and
therefore the difference between closed and open. (The photos are a simple view of
blood stasis in vessels near a wound, illustrating indirectly the
plasma-platelet-leukocyte events that are triggered by injury and which
become the basis for detecting the condition of “wound”.) Error signal is acute inflammation. Acute inflammation is that complex set of
acute phase cellular and biochemical events that is meant to defend the body
in conditions of injury and disease, then clean up the mess and prepare for
repair, then initiate the repair system.
As for every element on the control loop, this is another example of
countless items being rolled up into one black box. The individual effects of kinins or
interleukins or prostanoids are irrelevant to the control loop. As discussed on slides 24, 25, 27, 28, the
Wound Main Control Loop is an open model that allows any and all individual
elements of the entire system to be specifically added or exposed in order to
simulate their effects. However, the
core process, the Main Loop, is a collection of quintessential black box
control elements, and the aggregate condition of acute inflammation is the
error signal, with one origin or input (plasma-platelets-leukocytes) and one
effect or output (macrophages). One effect
or output? Yes, it is true that
inflammation as a whole has many effects on many events after injury (acute
defense, immunity, cleanup, etc.).
These operate as other controlled loops, either within the black box,
or else on parallel planes with inflammation being a point of intersection
between several regulated systems.
However, for the sake of driving the wound healing control loop, there
is just a single output, the effect of accumulating (integrator) and initiating
(transforming) macrophages from monocytes.
The photos illustrate the upper strata of the wound, the
topmost plasma protein inflammatory layer and its junction with the
gag-angio-attraction layer. Acute
inflammatory cells are present in the plasma, and so are some large
mononuclear cells (monocyte-macrophages) and some migratory spindle cells
(chemotropic angiocytes). The release
of platelet cytokines and related chemicals is how monocytes are transformed
to macrophages, establishing the link between the error detector and the
system controller. Note that the set of plasma-platelet-leukocytes is diagrammed as
the error detector whereas acute inflammation is diagrammed as the error
signal. Isn’t this self-contradictory,
redundant, or artifice? Isn’t
inflammation just the operations and consequences of
plasma-platelets-leukocytes? The
answer is that they are a pair. There
are actually three such pairs on the Wound Main Control Loop. These are all splits between anatomy and physiology,
between actuator and effect. First, there
is a tangible element that does something, then second there is the something
that it does. The three pairs are: (1) error detector & error signal
= detector (plasma-platelets-leukocytes, the mediators and controllers of
inflammation) and signal (the state of inflammation that they cause); (2) controller and control signal = controller
(macrophages) and signal (the growth factors they produce); (3) load and output = load (repair
cells) and output (the new stroma and epithelium that they produce). |
|||
|
|
|
||
|
This slide focuses on the mid cycle of the Wound Main Control
Loop, the system controller, the control signal, and the system load that is
actuated and controlled by the controller. Controller is macrophages.
Macrophages do not themselves heal the wound. As the system controller, they regulate the
repair machinery. Like any system
controller, macrophages are executives who are informed about the status of
the system and then issue orders for the workers to take some action. Macrophages start off in a standby form,
the blood borne monocyte. They are
informed about the status of the system by the error signal, inflammation,
which has the effect of transforming the monocyte. The error signal is issued by the system
error detector, and with respect to monocyte-macrophage transformation, it
comes in the form of platelet-released peptides such as PDGF. These transformative cytokines cause a committed
phenotypic change from the circulating planktonic monocyte to a local
amoeboid cell. As mobile phagocytes, the
macrophages have an afferent function to cleanup the injured area. As proliferation controllers, the cytokines
that they issue muster the local stromal cells to begin repairing the wound. From a biological point of view, it makes
sense that these two functions – (1) cleanup the mess and then (2) let us
know when you are done (initiate repair) – are integrated in one place, the
macrophage. With regard to the wound
control loop, the phagocytic cleanup functions of the macrophage are an
embedded or parallel loop within or intersected with the black box of the controller
block. It is the proliferative growth
factors that they make which feed forward as the control signal to drive the
next element of the loop. (The photos
show the macrophages, large migratory cells in the upper strata of the wound,
the plasma protein inflammatory layer, and the aminoglycan angio-attraction
layer. Being in the inflammatory zone,
they share the scene with numerous neutrophils. The macrophages themselves, as the system
controllers which regulate and attract repair cells, are the cause and target
of angio-attraction.) Control signal is growth factors. The macrophage controllers must issue their
orders to the system load, and they communicate by making peptide cytokines
and growth factors: IL’s, TNF, PDGF, VEGF, TGF, FGF, EGF, IGF, etc. These factors have a multitude of
regulatory effects on local events after injury, helping regulate the latter
phases of inflammation, and serving as the commands or initiators of repair. Along with their phagocytic functions, their
cytokines that influence late phase efferent inflammation can be rolled into
black box functions within or parallel to the control block. The control signal which drives the repair
loop is the set of potent pro-proliferative growth factors such as pdgf,
vegf, fgf, tgf-b, igf, egf which signal local repair stem cells or progenitor
cells to come to life. These
pro-proliferative cytokines and growth factors all have the usual functions
of such peptides – transformation and phenotyping, mitosis, and
migration. Transformation induces a
state of activity or phenotypic maturation in progenitor cells, allowing them
to become recognizable angiocytes or fibroblasts with specific metabolic or
structural functions. Mitosis allows
the activated progenitor cells to replicate and increase their numbers to
meet the needs of repair and stromal rebuilding. Migration is due to the chemotactic effects
of these growth factors and the chemotropic nature of the responder cells,
serving as a guidance system to bring the new activated repair cells to
precisely where they are needed. The
net effect of the control signal is to induce a state of histogenesis, in
which local responder cells will turn on to rebuild stroma and close the wound. The photo shows the effect of
macrophages to produce a chemotactic-chemotropic vector that draws activated
angioid cells into the wound. The
plasma protein inflammation layer is at the top, and below that is the full
length of the angio-attraction layer.
There is a background of neutrophils as expected. In the plasma layer there are large
amorphous mononuclear cells – the macrophages. At the bottom an organized vessel can be
seen. In between are vertically
oriented spindle shaped cells. These
are migratory angiocytes. They arise
from vessels below, activated by the mitogenic and chemotactic effects of
vegf and other angiogenic cytokines.
They migrate through the intervening aminoglycan layer toward the
source of the angiogenic stimulation, the macrophages above. Controlled load is the set of responder cells which,
commanded by the controller (macrophages), will do the actual work of trying
to restore the system and correct the system error. These are the local mesenchymal cells –
angiocytes first, then histioblasts, and lastly epithelium – which will
restore the damaged stroma and close the wound. These cells exist normally in a standby
form, either as passive stem cells awaiting the call, or else as mature
functioning components of existing stroma that are still capable of
proliferation or pluripotent re-differentiation. There seem to be 3 sources of the
regenerative new mesenchyme. The stem
cell reservoir of regenerative angiocytes and fibroblasts has long been
ascribed to angio-pericytes, the perivascular cells. In recent years, the honor has also gone to
circulating presumably marrow-derived pluripotent stem cells. There certainly is evidence that both play
a role in repopulating the healing wound.
However, one need only to look at any H&E or trichrome stain of
any random wound biopsy to realize that a preponderance of regenerative cells
come from existing angiocytes in nearby normal blood vessels. In the normal tissues subjacent to the
wound, angioid cells all become hypertrophic with cytoplasm and nucleoplasm,
an effect of pro-proliferative pro-mitogenic pro-migratory angiogenic
factors. Angiocyte mitoses are easy to
find in or near vascular walls, and the peeling or breaking away of
angiocytes from existing vessels as a prelude to their spindle-shaped
migration is abundant. The origin of
fibroblasts between coalescing vessels in the mid strata of the wound is not
so obvious. Remember that there is
extraordinarily little biological difference between angiocytes and
fibroblasts and myofibroblasts, and they can share or trade phenotypes. For example, in conditions of tissue growth
and vascular adaptation, new vessels begin as minute capillaries of just one
or a few circumferential cells. As
they grow, mature, and develop a larger angiosome, they must get a progressively
larger diameter and thus they must also get thicker (Wolf’s and Laplace’s
Laws), eventually getting a muscular media and elastic lamina, etc. It is all the same cell doing these
different tasks, taking on these different avatars based on local signals,
promoters and inhibitors, based on the principles of parsimonious
self-organization (see Part 3).
Perhaps new angiocytes in the healing wound derive largely from
existing angiocytes, whereas fibroblasts are more likely to come from
pericytes and circulating stem cells.
Perhaps they all contribute equally.
This is where conventional bioscience is necessary to answer
unresolved questions. What is
important from a control point of view is that the system controller issues a
control signal, and any and all potential responder cells that happen to be
there (or are chemotactically attracted there) can then respond. The photos show the assembly of new
stroma as a consequence of the system load responding to the controller. The upper photo shows mid-level wound
events in the histio-attraction layer, where young fibroblasts are making
young collagen and matrix interspersed between organized but still immature
vessels. The lower photo shows events
deeper in the histio-organization layer, denser flattened fibroblasts packing
the space with fibrous collagen. |
|||
|
|
|
||
|
This slide focuses on the back end of the Wound Main Control
Loop, the system output created by the controlled load, and the way that this
feeds back into the system comparator. Output is the proliferative part of the wound module, the tangible
substance and structures that result from the activities of the system
load. Remember, from a control point
of view, it is the responder cells, the active agents of production, that are
the load that must be controlled. The
system output is the stuff that they make – fibro-vascular matrix and wound
closure. Specifically, the load cells
produce (1) restoration of mesenchymal stroma via aminoglycans and ground
substance, then vessels, then fibrous matrix, and (2) wound closure and
mesenchymal sequestration via contraction and epithelialization. (The photo shows a histological view
of the wound at the level of the histio-attraction layer, demonstrating all
of the mesenchymal components of system output and tissue building. The other photo is a gross view of the new
stroma – “granulation tissue” – plus the signs of closure – contraction and
epithelialization.) The generation of
output products directly modifies the state of the system, i.e. more tissue
and less wound. This has the dynamical
effect of bringing the system back toward the system reference. That of course is the whole purpose of any
control loop, to drive a perturbed system back toward reference. As the load cells create output which
corrects the variances in the system, the discrepancy between actual and
ideal state, as measured by the error detector, will diminish. As the error diminishes, the error signal
and the driving force in the loop starts to diminish, eventually ceasing when
the discrepancy is zero. In this
explanation of the Wound Main Control Loop, all that remains to complete the
loop is feedback, some way to report the current state of the system back to
the system comparator. Feedback is the open wound, i.e. some detectable and
transducible set of properties of the state of “openness”. Recall from the discussions on slide 20 the
differences between the ways that the system comparator senses the feedback
and the reference. System reference
(normal closed tissue) is a passive condition. The error detectors
(plasma-platelets-leukocytes) simply “ignore it”, meaning that they are not
activated by any of the properties of normal tissue – which is of course the
way that they are tuned – plasma, platelets, and leukocytes do not activate
when the are contained in healthy uninjured blood vessels and unstressed
tissues. What the comparator elements
are actively sensing are the byproducts of injury and inflammation and openness
– these are the conditions which trigger or activate plasma, platelets, and
leukocytes. Specific activating stressors
or triggers include hemorrhage, plasma leak, stasis of
blood-plasma-platelets-leukocytes, necrosis, bioburden, fluid and hydration
changes, vascular reflexes, etc. How
it is that these conditions initiate and auto-amplify thrombosis and
inflammation is the stuff of conventional bioscience and need not be reviewed
in depth. What is important from a
control point of view is that any open wound will have some degree of any or
all of these attributes, and these are the conditions which activate or
perpetuate plasma, platelets, and leukocytes.
Thus “open wound” is a positive input on the system error detector,
whereas healthy and healed is a neutral input. For the system comparator and error
detector, an open wound and its attributes are always a detectable discrepancy
from normal, and the discrepancy elicits a response, the error signal of
active inflammation. As long as there
is “open wound”, there is a positive error signal, and the loop keeps driving
until the error is gone, meaning no more “open wound”, i.e. healed. (The photos show a healthy wound
actively healing. The progressive
closure is a consequence of output from the load cells, but until it is fully
closed, there will be conditions which feed back to continue driving the
process.) The Wound Main Control Loop is an abstraction of the real
process of wound healing. It is a
means of distilling an extraordinarily complex physiological process with
innumerable players into a valid conceptualization that clearly, simply, and
precisely explains the core dynamics of the system. As will be shown on subsequent slides, it
is also an open model that maintains a place for all of the biological
realities of the system. It is crucial
to remember that for complex systems, conventional biosciences are required
to discover and characterize individual components of the system, but
dynamics – physics – is required to understand how the many elements
inter-operate. The loop is autonomous. It starts and stops correctly depending on
the circumstances of normal tissue versus open wound. Once an injury or wound is closed and
reorganized, the control loop ceases to run.
However, the inherent machinery and dynamics are still there, in an
unseen quiet or standby state ready to reactivate if new injury or wound
occurs. Note that when the wound is
epithelialized and nominally healed, that related biological activity is not
yet fully finished. The restored
tissue is not fully reorganized until it is completely matured, a process
which will take months or years. Maturation
is also a regulated process, but it operates sequentially after the main
wound healing process and control loop are settled. Thus, maturation can be seen as a parallel
or sequential process, important biologically, and intersected but otherwise
separate from the Main Loop. The photos
are the same as those on slide 3, reminding that normal skin does not
spontaneously start healing, and that a healthy wound will go through the
process properly until healed, and then the process ceases. Finally, notice some of the key dynamical attributes of the
Wound Main Control Loop. This system
is self-organizing – as the loop runs, tissue reassembly is automatic and
correct. The loop and wound healing
are purely reactive – they do not start and stop of their own initiative. Rather, they start and stop only in
response to explicit trigger conditions.
The loop and wound healing are reference or error driven – they
operate only as required to correct a variance from normal. Thus, assuming that the process is healthy,
it will accurately restore the injured tissue to normality. Reactive self-organizing error-driven
dynamics are of vital importance to the health of the system and host. This is true for all systems with these
attributes, especially for life-and-death biological systems, as these
properties confer robustness and fault-tolerance against the noise and
perturbations of normal variability and extrinsic stresses. |
|||
|
|
|
||
|
There are many reasons for modeling or abstracting a complex
system. Among them are being able to
observe and study the behavior or output of the system, and being able to
understand what is wrong when the system varies from normal. For the moment, we will leave wounds behind
and return to control systems in general.
There are certain types of generic responses or behaviors that all
controlled and non-linear systems will
potentially exhibit, and we will look at them here. Remember the basic purpose of control. Systems need to be at a certain value or
within a certain operating range in order to function as intended, or the
system needs to maintain a certain state or value for the sake of its output. Once a system is at a desired value, it might
not stay there. There may be decay or
drift of intrinsic parameters, or it may be perturbed by extrinsic
forces. A control system monitors
itself. If it senses variances from
reference, it tries to correct them and restore the system to the reference
value. The system is trying to counteract
changes, pulling back in the opposite direction of the unwanted change. If the system drifts low, the control loop
pulls up. If the system is pushed high,
the control loop pulls down. The
control loop is always trying to reverse the direction of unwanted variances,
trying to keep the system as close as possible to the reference or target
level. Ideally, these corrections are
made as quickly and as smoothly as possible.
However, there can be errors or instabilities in the correction
itself, leading to over correction, under correction, or a variety of
dynamical behaviors. Whether or not
the system behaves as needed depends on the quality and tuning of the
circuit. In engineered systems, man
made machines can deliberately be designed and tuned to have specific types
of responses. In natural and
biological systems, the quality and tuning of the responses represent
evolutionary adaptations based on principles of thermodynamics and non-linear
dynamics (see Part 3). Here is a
sampler of possible generic responses in controlled systems. One-shot. An
incidental perturbation or trigger suddenly knocks the system to a new
value. The change from baseline is
corrected by a smooth return to reference or baseline - convergence. The dynamics or mathematical nature of the
return can vary based on the nature of the system. The decay could be at a constant rate
(zero-order, linear), or at a rate proportional to its current value (first
order, exponential), or with some other dynamic. First order exponential decay is probably
the most common dynamic in natural systems (chemical, electrical, mechanical,
physiological, etc). This will appear as
the typical curved asymptotic graph illustrated. This is the ideal response to perturbation,
the way engineers would want to make most machines behave. Common examples from biology include the
serum concentration of a drug after a one-shot injection, the relaxation of
tension in a stretched scar, and the response of a healthy wound to simple injury. Normal wound healing, its aggregate closure
as well as many individual components, and especially the overall dynamics of
the Wound Main Control Loop, will exhibit this response to the initial
injury, assuming that the system is healthy and that the injury was a
one-time instantaneous event. Saturation-extinction.
Under certain circumstances, control may fail or be overwhelmed. (1) The intensity of the perturbation can
drive the healthy system out of bounds of its operating range (if you will, the
extrinsic disturbing force is greater than the strength of the system to
respond). (2) If the system is
intrinsically inadequate or has become faulty, then it cannot respond as
designed to forces which would otherwise be within the response range. The effect is that the system fails at its
extrema, reaching its maximum physical capacity to respond, or exceeding its
operating range and design limits - divergence. It could go into saturation, failing at the
upper or loaded end of the range, or it could go into extinction, becoming
zero. Either way the system was meant
to be operating somewhere in between, and it has now been forced up or down
out of the operating range. Since the
system is no longer in its operating range, it can no longer function and
respond to the altered state, and thus it cannot correct itself. The system will then remain in its
saturated or extinguished state until the perturbation is relieved (or the
system machinery is repaired). As a
biological example, consider a patient with congestive heart failure,
cardiomyopathy, and coronary artery disease who is currently compensated and
free of acute symptoms. Suddenly the
patient goes into florid failure due to a hypertensive crisis or the onset of
pneumonia or peritonitis. Sudden changes
in afterload or cardiac work and preload will change many parameters such as
myocyte fiber length, ventricular diameter, ventricular dp/dt, etc., i.e.
those physiological parameters that must be properly “tuned” if the
myocardium is to remain in its proper operating range and be an effective
pump. Even oxygen delivery and energy
utilization are important for the myocardium to function effectively, but
those parameters are impaired because of the coronary artery disease. See what is happening? The acute extrinsic stressors result in
decompensation of fiber length and ventricular diameter which push the pump
out of its operating range. The
coronary artery disease is loss of an intrinsic component, loss of a “degree
of freedom” in the system’s innate ability to react and compensate. As the failure begins, conditions which
provoke failure get even worse (a non-linear amplification), and the
decompensation progresses until there is no chance of the system ever pulling
itself back into the proper operating range.
For any specific parameter you are looking at, they are all out of
bounds, either high or low, saturated or extinguished. If the pump is physically damaged e.g. by an
infarct, then corrections may be impossible.
However, if the machine stays inherently undamaged, then interventions
to restore the proper operating range will allow the system to come down from
its saturated state and start once again to actively control and properly
regulate. Treating hypertension or
volume overload or systemic metabolic load or correcting coronary artery
occlusion are the types of interventions which will be needed to pull the
system back into operating bounds. As
a wound example, saturation is the response to severe inflammation or active
disease. Under conditions of active
disease and ulceration, the repair process cannot prevail over the destructive
events. The repair process and control
loop become saturated – on full time “overdrive”. What is happening is that the error
detector sees a widening discrepancy between normal and actual state, so the
system gets driven to maximum capacity.
However, the control loop is “blocked” or “open circuit” at the system
output stage, because output is being suppressed or destroyed by the active
injury and inflammation. No output
means no correction of the system state.
Therefore “open wound” feedback does not diminish (in fact it gets
worse due to disease and inflammation).
The system error persists and the front part of the control loop
remains maximally driven and operating at its peak physical capacity, i.e.
saturated. So, the system is
saturated, but nothing productive happens.
Alleviating the active disease and injury will allow the control loop
to reenter its operating range. Amplification-attenuation.
The response of the system to perturbation might be qualitatively
correct but quantitatively incorrect – too much or too little. If the response is amplified or
exaggerated, then there will be an over-correction. If the response is blunted or attenuated,
then there will be an under-correction.
If the loop continues operating, there will be subsequent
opportunities for the system to correct itself, but if the imbalance is
system wide or consistent, then it will repetitively miss the mark. Biological wound examples that seem to fit
this dynamic are pyogenic granulomas (see slide 28) and keloids, in which the
wound healing process is amplified, exaggerated, and overshoots the intended
target, making too much wound healing and too much new tissue. Damped sine. A
controlled system needs time to sense errors and actuate corrections, and the
corrections need time to propagate and feed back. The consequence of such time delays is the possibility
of over- or undershooting the target, or getting oscillations. When the controller initiates a correction
(implemented by the load), that correction will have a certain velocity
(speed and direction), a rate at which the system state changes in the
desired direction. If the rate is perfectly
tuned, the system will settle on the correct value. If the correction is a bit too fast or
strong, it risks overshooting the target.
If it does overshoot, this generates an error condition of the
opposite sign, and the control system then tries to bring the system back the
other way. If it could all be sensed
and actuated instantaneously, the return to baseline would always be
smooth. However, because of time
delays, the controller is unaware of the overshoot until it has occurred, and
any corrections it then makes will need time to kick in, allowing the system
to continue moving the wrong way before it can start moving back the other
way. Imprecision and delays will be
consistent, so the same thing happens going in the other direction. The net effect is that the system “rings”,
a transient oscillation as it decays back to reference, The peak-to-peak envelope (or else the
root-mean-square values) will be the expected exponential decay. This oscillation shaped by the decay is a
damped sine wave (or a damped whatever if the oscillatory waveform is
something else). This is the brief waveform
you might see on the screen when you turn the power off to a conventional CRT
display (television, monitor, oscilloscope, etc). It is also the transient wiggling in a
spring that is stretched and released, and the slowing of a pendulum due to
friction. Damped sine waves are seen
in biological systems in somewhat arcane laboratory circumstances, usually
related to neurological, sensory, and certain cellular and chemical events,
i.e. micro-scale physiological systems.
If you work with spine injured patients and have a chance to observe
the reflex spasms that occur briefly in response to stimulation, that is a
perfect clinical example of a damped sine wave as the spasm triggers then
decays. Oscillation (good).
Not all oscillations are bad, and not all are transient or
damped. Oscillation is in fact the
goal and healthy productive state of many systems. The principles of feedback, reactive
correction, overshoot, and timing and delay can all be engineered and tuned
in such a way that the back-and-forth over-correction under-correction
becomes a sustained oscillation. Of
course, all systems have energy losses which will damp an oscillation, so for
it to be maintained, new energy must be repetitively restored to the system,
and when it is, oscillation can be sustained indefinitely. These principles allow us to build clocks
and radio wave circuits and engines and all kinds of things. Biological examples of autonomous periodic,
i.e. oscillatory systems include a bee’s wingbeat and a heartbeat. While overt fixed rate oscillations are not
evident in wounds, these principles do apply to issues of thrombosis and
inflammation and the sustentation of necrosis and ulceration, as explained in
Part 2 of this series, “Auto-Immunopathy and the Intrinsic Disease of Wound
Healing”. Oscillation (bad).
In mathematics, all periodic or harmonic (oscillatory) functions can
be represented by a polynomial of sines and cosines (a Fourier series). Engineers use this principle to combine
different frequencies to create any arbitrary waveform. The same principle works in reverse to get
information out of combined waveforms, such as decoding a particular channel
from all of the information that comes through a single television
cable. So, multi-harmonic oscillation
can be a good thing, but when it shows up unintentionally, or it is
exaggerated, or it is the result of unintended subsidiary circuits and
feedback loops in complex systems, then it is unwelcome and can be the bane
of good control. All clinicians are
familiar with the concept from looking at ECGs that are blurred by 60 Hz
crosstalk from nearby electrical devices and wall power. As an impairment of control in biological
systems, Parkinson’s disease and cerebellar and other tremors are examples of
inadequate or delayed control resulting in problematic single or multi
harmonic oscillation. Chaos. When systems and
circuits and machines seem erratic and unstable, the problem could be one of
unwanted oscillation, or it could be one of noise and random
variability. However, variability in
multi-control systems is usually due to something else – chaos. In fact, if you measure any complex natural
multicontrol system carefully enough, especially biological systems, you will
find that even repetitive functions like breathing are not strictly fixed
rate sinusoidal oscillations. The
behavior of complex systems can at times seem very erratic, non-harmonic, and
non-analytical (not reducible to conventional functions of algebra and
calculus). Yet at the same time, these
systems are following precise physical rules and principles. This is chaos, the behavior of complex
non-linear systems. “Chaos” is an
unfortunate term because in physics and math it does not mean the same thing
that in means in the vernacular vocabulary.
What it means is that systems can behave in seemingly complex ways
that cannot be described by simple harmonics or other equations, nor are they
random and noisy. Chaotic systems are
highly deterministic and rule-driven, and highly organized and
structured. The trick is that you need
to know how to look for that structure, and how to describe that structure if
the face value data stream is to make any sense. Most chronic wounds are chaotic – i.e. they
have chaotic dynamics, how they behave over time. The origins of chaos in the wound are
introduced on slide 32, and chaos is explained in detail in Part 3,
“Chronicity and the Physics of Wound Failure”. If you are an electrical or a mechanical or an aeronautical or a
control engineer, you are apt to deal with any and all of the types of
responses and instabilities described here.
Examples have been given of how all of these behaviors can be seen in
controlled biological systems (and remember, virtually all biological systems
are controlled). However, any system
has its own set of physical and dynamical realities, and consequently its own
characteristic ways of failing or misbehaving. For wounds, not all of these responses
apply. Oscillatory or harmonic behaviors
are not relevant. We often see
amplification and attenuation in response to various therapies that are applied,
but this rarely changes the intrinsic dynamics of a given wound. The core dynamical behaviors and
misbehaviors that are a part of everyday wound physiology, pathology, and
clinical management are three:
one-shot convergence, saturation-extinction-divergence, and
chaos. As described in explicit detail
on subsequent slides and in Part 3, the healthy wound has a normal
one-shot response to the injury, converging on the desired state of
“healed”. The sick wound,
subject to active disease and injury, is diverging, its controller saturated
or extinguished as it becomes progressively inflamed, necrotic, and
ulcerated. The impaired wound
which is not actively sick from acute injury, but which nonetheless will not
heal, is chaotic. Part 2 of this
series will explain the biological basis for that chaos, and Part 3 will
explain why, once that state has occurred, it can be difficult or impossible
to get the wound to behave otherwise. |
|||
|
|
|
||
|
Healthy healing due to one-time injury follows simple one-shot
dynamics. Both the displaced values of
system parameters and the overall dynamics of the loop will settle smoothly
to baseline or pre-morbid values if the system and its components are healthy
and there is no perturbation by outside forces. What happens though if the
wound is sustained by disease or repetitive injury? How will the control loop behave, and what
will a graph of the system state or dynamics look like over time? As for any properly abstracted model, the
Wound Control Main Loop is an open kernel that can be amended and appended,
opened up and plugged into. For the
sake of modeling real wounds, any parameter, sub-parameter, physiological
reality, pathological challenge, and clinical variance can be represented and
analyzed on the loop. We will now look
at how the chronic active wound, sustained by ongoing disease and repetitive
injury, can be represented by the loop, and how the dynamics of the loop will
change due to sustained stressors. Active injury:
The top panel shows the most trivial amendment to the Wound Main
Control Loop, how to represent sustained exogenous injury. The effect of the loop output block is to lessen
“open wound”. Active injury does the
opposite, it creates more open wound.
These two effects obviously counteract each other, adding or
subtracting from the state of the wound.
To represent this, injury is summed with the system output, introduced
at the node shown. The composite
effect of wound healing (less wound, negating effect [-] on “open wound”) and
new injury (more wound, additive effect [+] on “open wound”) determine the
net state of “wound” which is then the feedback into the error detector which
then decides how hard to drive the loop. Inflammation 1:
Acute inflammation is the proverbial “double-edged sword”. Inflammation induces wound healing, but
inflammation also inhibits healing.
How, why, what, huh? First,
recall that acute inflammation induces the wound module – this is the normal
physiological sequence of events and the trigger that turns wound healing on
after injury. However, inflammation is
just a big “black box” event with many players, roles, internal operations,
and intersections with control loops other than wound healing. It is not “inflammation” per se, its
aggregate or many activities which turn on wound healing. Rather, it is just one component of
inflammation that has that task, the macrophage. So, yes, it is true that inflammation
induces wound healing, but . . . Acute
inflammation is inherently destructive and inherently inhibitory. It is meant to destroy non-autogenous
foreign biotes and chemicals. It has a
variety of mechanisms via enzymes, ionic radicals, and cell killing
activities that will chew up your own ground substance, connective matrix,
and cells as readily as it chews up the invaders. In fact, normal healing cannot begin, the
stroma reconstructed, until the injured area is first deconstructed in
preparation for repair. This is all
just the normal set of afferent wound events.
One of the effects of acute inflammation is also to directly and
explicitly inhibit the repair process.
Many of the acute phase chemicals and mediators of inflammation have a
direct inhibitory effect on many of the reparative events, such as angiocyte
and fibroblast proliferation, that are yet to come. Teleologically, this is a beautiful
mechanism that keeps the body from wasting its repair resources until the
time is right. Dynamically, it allows
the integrated system of injury recognition-response-repair to function
correctly with proper short term dynamics and without long term adverse
sequelae. These issues are discussed
on slide 32, and then at length in Parts 2 & 3. Simply put, the inflammatory phase of a
response to injury is itself a one-shot ramp-up then decay of defensive
activities that keeps the repair phase suppressed until the right time. As acute inflammation wanes (assuming a
single one-shot injury event), inhibitors of the next repair phase diminish,
allowing repair to turn on. However,
repair also depends on an active promoter, and the period of inflammation has
allowed the numbers and effects of macrophages to accumulate. Pro-repair regulators build slowly but
steadily in the acute wound, whereas initial or recurrent acute phase
inflammation has a more immediate effect to suppress repair (which is why it
must abate for active repair to occur).
From a mathematics and dynamics point of view, these events sound like
typical integrator and differentiator functions. To model this duality of acute inflammation (middle panel) we
can introduce a split, a bifurcation in the wound circuit at the inflammation
error signal. An integrator gradually
builds macrophage mass, for robust healing at the right time, the bridge
between the inflammation one-shot and the wound healing one-shot (see slide
32). The parallel differentiator
transiently suppresses active healing during “flare-ups” of acute injury and
inflammation, keeping the body from wasting resources (and minimizing the
chances of exposing endocellular materials to possible auto-immunization –
see parts 2 & 3). If you are
unfamiliar with basic principles of electricity, the circuit elements shown
are two basic types of R-C resistance capacitance circuits. The green circuit feeds the error signal
through a resistor, and a capacitor bridges the output to ground. Resistors impede – slow down if you will –
the flow of electricity. The function
of the capacitor is to accumulate charge, preventing it from flowing through
the rest of the circuit until the capacitor is full. The net effect is that if a signal or
voltage appears at the head of the resistor, it will take time for that
voltage to build up on the tail end of the resistor. The build up of voltage is a logarithmic
rise or fall which mathematically is the timewise integral of the
current. Thus this circuit – the
voltage on that capacitor – is an analog integrator. Also, capacitors have their own impedance
which lessens as frequency gets greater.
Thus, for currents flowing through this circuit, high frequency
information will get shunted to ground before getting to the next block,
whereas low frequencies will have time to develop their voltage on the
capacitor, delivering that information to the next block. Thus this circuit is an integrator but also
a low-pass filter – low frequency information gets through to the next stage,
and high frequency information vanishes.
The red circuit is the inverse, feeding currents through a capacitor
which is shunted to ground through a resistor. Voltages applied to the head of the circuit
will be immediately transmitted across the capacitor, but as charge builds
up, the capacitor gets saturated and current stops flowing. Thus the waveform will be one of a voltage
spike that then decays logarithmically as charge on the other side of the
capacitor flows out through the resistor.
This is the mathematical as well as the physical inverse of the green
circuit, making its output voltage the first derivative of the current. Thus, this circuit – the voltage on that
resistor – is an analog differentiator.
A corresponding effect is that high frequency information will get
across the capacitor, but lower frequencies are stopped as the capacitor has
time to saturate. Therefore this
circuit is differentiator but also a high-pass filter. What does all of this have to do with the wound, inflammation,
and the wound control loop? Everything. Remember, characterizing the
“nuts-and-bolts” biology of these systems is the stuff of the conventional
biosciences. Understanding the
dynamical operation of the wound (or any complex) system is physics. There are of course no electrical
components in the wound loop, but electrical components are frequently used
as ways of demonstrating the nuts-and-bolts structure of various physical and
dynamical elements when modeling systems.
We could of course just replace the electronic diagrams with simple
blocks containing an ò or ¶ to denote integrator and differentiator. What is crucial to remember is that however
we choose to represent these elements and events, it is their interoperations
and dynamics that are important. To
reiterate, acute inflammation causes the buildup of macrophages, a low
frequency integrator function that in the healthy wound ensures the one-shot
coupling of inflammation to the reparative wound module. However, sustained or repetitive or
recurrent acute inflammation will have an immediate inhibitory effect on the
wound by negatively affecting the controlled load, the actual repair cells. This suppression is a high frequency
differentiator function that ensures that repair resources are not wasted in
the early inherently destructive phase of response to the injury. Inflammation 2:
The defensive and destructive aspects of acute inflammation do more
than just inhibit or delay repair. By
its very nature of being destructive, it is injurious to the host matrix or
tissue. With sustained injury and
pathological wound conditions, acute inflammation is the proximate mediator
of active ulceration. Clinically, this
is especially evident in suppurative and in autoimmune ulceration where the
inflammatory-lytic pattern of active ulceration is overt and aggressive (see
Part 2). The ulcerative effects of
acute inflammation can be modeled by summing the immediate defensive
“differentiator” limb of acute inflammation into the injury node. |
|||
|
|
|
||
|
As a further example of how the Main Wound Control Loop is a
comprehensive open model that can accommodate all aspects of the wound, we
will now look at the role of wound therapies and clinical interventions in
affecting the operations and dynamics of the loop. Basic wound therapies:
The first panel demonstrates the effects of basic wound therapies
treatments. These are the general,
diagnosis non-specific, common treatments that are the first and mandatory
set of therapies that control a wound, arresting its progress, alleviating
symptoms, and generally ensuring the safety of the patient. These include basic wound hygiene and
debridement, topical care, and control of inflammation and edema. Each of these interventions counteracts
some detrimental aspect of the wound, host, or injury that can be represented
as a component of inflammation. To
illustrate these ideas, we start with the “Inflammation 2” panel from the
preceding slide, and then we open up the inflammation “black box”. Within the box, four aspects of
inflammation have been selected to illustrate these dynamics: edema, chemical
mediators, thrombosis, necrosis.
(These four are important aspects of inflammation, but they were
selected arbitrarily and limited to just 4, so as to keep this illustration
simple.) Each of these inflammation
mediators is shown feeding into a two-input amplifier (grey triangle, direct
and inverting inputs), paired with a “red cross” therapy which corrects
injury, maintains hygiene, controls debris, inflammation, & edema, etc. The therapies counteract the pathologies,
and depending on which has the greater strength, the output of each amplifier
will flip positive or negative. All of
these individual elements with their counter-therapies then sum through
output filters ƒ which determine the net value of the state of inflammation. The two output stages are inverses of each
other (the circle input is a negation or inversion). Depending on the net state of acute active
destructive inflammation versus settled treated conditions, one or the other
limb will be driven, either the integrator function which builds the system
controller (the treated state, leading to active healing), versus the
differentiator function which inhibits healing (the acute inflammation state
of protect-the-host-but-suppress-repair). Wound healing therapies:
Wound healing therapies are the technological products and modalities
used to stimulate repair after initial “red cross” therapies have controlled
injury and inflammation. These
treatments directly effect the intrinsic engine of repair – the efferent
elements of the wound module (controller and load cells) and the dynamics wound
healing control loop. Three examples
are shown. (1) Exogenous cytokines
include pharmaceuticals such as PDGF, and neonatal living cell therapies. These either mimic or go beyond natural
macrophage activity to make pro-proliferative growth factors. As such, they are acting as off-the-shelf
wound controllers or control signals.
They are represented by summing into a node that combines with the
native macrophage growth factors. (2)
Skin grafts and other wound repair operations have the effect of directly
restoring epithelium and immediately closing the wound. They have the same effect as “system
output”, the proliferative tangible “stuff” which restores tissue and diminishes
“open wound”. As such, they are
represented by summing into a node that combines with the output. (3) Biologicals are non-living
biologically derived materials that are meant to be used as skin substitutes
or temporary wound closure. They also
include non-biological materials that are engineered to serve the functions
of skin and biological covers. These
materials do not restore actual living epithelium, but as skin substitutes
they have a comparable biological effect (even if only of limited
duration). They do not create genuine durable
living “system output”, but they do have
the immediate effect of diminishing the state of “open wound”. As such, they are represented by summing
into a node that combines with the system feedback, thereby reporting to the
system that the wound is acting as though it is closed. |
|||
|
|
|
||
|
On the last few slides we saw how we can diagram the wound
control loop to account for perturbations and altered states and other variances
from normal healing, including how therapeutic interventions can fit into the
system. A diagram is one thing, but
the goal of having it is to understand how the system actually operates and
responds, what the output of that diagram will look like, and how to use it
to understand the behavior of the system.
Here we start to look at what happens when the wound is stressed or
pathological, i.e. how the core loop (or the core with circumstance specific
amendments) reacts to normal and abnormal conditions. Keep in mind that even when the wound seems
to be in whacky and crazy or frustrating or exasperating states of
misbehavior and non-healing, that does not necessarily imply that the wound
healing machinery is broken, The main
loop and its physical-biological components are meant to respond to inputs
and perturbations, and if the inputs get crazy, then the responses can seem
crazy too. As will be seen in detail
in Part 3, the wound control loop and its core elements rarely get inherently
sick. Rather, their responses to
complicated sustained adverse inputs can become disorganized. On slide 23, we looked at generic responses
of controlled systems to perturbation.
The kingdom of biology has examples of all of those types of response,
such as harmonics and oscillations, but for wounds and most other biological
systems, controlled behavior tends to fall into three general responses or
attractors. Convergence: This is the healthy wound. The dynamics are a one-shot. Injury or disease suddenly “jumps” the
wound up to an elevated state. From
there, the control system brings it back down to normal (healed) along a
simple trajectory of “decay”, ideally a smooth return from open and disrupted back
to closed and reorganized. Divergence: This is the sick wound. Primary disease and injury are at work,
causing active necrosis and ulceration by thrombo-infarctive and
inflammatory-lytic mechanisms. The
control loop is either overwhelmed by the strength of the perturbation, or it
is suppressed by one means or another, and the wound rises into the
undesirable dynamical state of saturation, a “full blown” actively pathological
wound. Bringing the wound back into
the operating range of the control loop, where individual components of the
control loop are not overwhelmed or suppressed, where the wound healing
machinery can then operate to heal the wound, requires deliberate active treatments
to abate the disease and injury. No
magic wound potions and no legitimate wound healing therapies will succeed
when the wound is acutely sick and inflamed.
There are plenty of tangible chemical, cellular, biological reasons
for this as well, but as a dynamical system, it is also easy to see how a
saturated system has to be brought back within the operating range of the
repair system before it can work.
Clinically, that means first things first: control disease, injury,
and inflammation, and only then when they are controlled can you move on to
step 2, discretionary therapies to make the wound heal, or make it heal
faster. Chaos: This is the impaired wound. These are the wounds from slide 2, the
premise of this talk. Exam-to-exam,
for many months, there is no net change in the wound. It does not necessarily get any worse when low
levels of underlying disease are alive, and it certainly does not get any
better in spite of a wide range of diligent treatments. The control loop “orbits” on an attractor,
going back and forth a bit, but never getting too far, and from which it can
escape only with therapies that strongly regulate or drive control nodes. |
|||
|
|
|
||
|
The last slide looked at wound pathology from a dynamical sense,
looking at the attractors or generic dynamics that can occur in healthy and
sick wounds. Here, we look at how the
control loop can be adjusted to account for specific diseases or clinical
disorders. Here are three examples of
“open circuit” wounds in which the control loop is interrupted in one way or
another, thereby arresting wound healing until the open circuit is reclosed
or restarted. 1 - Radiation wound = blown fuse: High dose radiation damages cells in ways
that keeps them from dividing. That is
its therapeutic intent when used for cancers, keloids, heterotopic bone,
etc. However, the same effect on
stromal tissues does latent damage to local progenitor cells, inhibiting
their ability to have a proliferative response to injury. Mesenchymal responder cells, normally in
wound healing standby mode, are rendered incompetent to do their job if
eventually needed. With responder
cells gone, the controlled load in the system is missing, and the loop is
thereby opened at that position. The
effect is modeled as a blown fuse rather than an open switch. The difference is that switches can be
flipped on and off, whereas a blown fuse is an irreversible fault in the
circuit which can be corrected and the loop restarted only by replacing the
damaged component. The example shown
is an ankle ulcer after 6500cGy for mycosis fungoides (cutaneous
lymphoma). Prolonged non-healing was
corrected by transplanting wound competent cells back into it from a donor
wound. This was done by implanting a
perforated plastic chamber under the skin in a healthy part of the body
(abdomen in this case). Two weeks
later, the “granulation tissue” wound module within the chamber was harvested,
then minced-trypsinized-suspended, then injected by syringe and needle
throughout the wound. The top photo is
the appearance at two weeks, with granulation tissue starting to appear. A second harvest and transplant was done at
that time, after which 100% of the wound proliferated, and then a skin graft
was placed. The lower photo is the
healed result a few months after the process was started. 2 - Chemotherapy = open switch: This is another ankle ulcer in a patient
with a myeloproliferative disorder being treated with hydroxyurea. The ulcer would start and stop in synch
with cycles of the drug. Unlike
radiation which damages the proliferative cells, thereby damaging the load
element, antimetabolite drugs have only a transient effect to diminish the
metabolic output of the responder cells.
Thus it is acting as an on-off switch.
Although its immediate biological effect is on the load cells, its
dynamical effect is on-off for the output element of the control loop. The two photos show the progress made
during one cycle of drug = off and healing = on.) 3 - Shear = ground out:
One of the most important principles of mesenchymal biology is its
ability to respond to mechanical force – compression, tension, shear. Under load, mesenchyme responds or
differentiates in such a way as to resist the load, thereby minimizing
stresses or strains in the material.
This is a crucial part of normal musculoskeletal and cardiovascular
embryology, histogenesis, and organogenesis.
It is also the principle that governs much of the pathology, sequelae,
and care of countless problems in clinical medicine. In its earliest enunciation and best known
form, it is Wolf’s Law – compression begets bone, tension begets tendon, etc. With shear, the need to minimize stress
within tissues is corrected by having the stroma (fibroblasts and angiocytes
and the structures that they make) undergo synovial or serosal metaplasia. This is a response that assumes that motion
is anatomical (tendons, joints, bursas, coelom, etc). The healing wound is nothing more than
active biologically plastic mesenchyme.
When shear is present, normal wound healing is simply shut down as the
system is shunted off to a physiological “program” of synovial metaplasia. Of course, that is the teleological way of
seeing it. The reality is that there
is no difference in this situation between “healing” as we envision it and
the serosal metaplasia. It is just
mesenchyme – fibroblasts, etc. – responding to the applied inputs and cycles
of the control loop. When shear is
present, the young mesenchyme rearranges itself into mature flat fibrous
lamellae and then goes to rest so that no adhesions form between surfaces
that were meant to move. (It is also
the shearing of blood flow against the angiocytes in forming blood vessels
that matures the angiocytes and “solidifies” their aggregation into formal
vessels.) In embryology, this process
is essential. It also works to your
advantage when you are trying to rehabilitate a tendon or joint, but it works
against you when you are trying to make flaps heal on their tangential
surfaces or when you are trying to get skin to heal over a moving
tendon. Shown is an EDC tendon across
ankle (extensor digitorum communis). The
ink marks show the range of tendon gliding with active ankle motion. The wound surfaces have either normal
tenosynovium or else only marginal signs of wound healing. Observe on the left that the fully exposed
surface has slight amounts of angiogenesis, etc., i.e. granulation
tissue. On the right, with the tendon
transposed upward, the areas that are shearing directly against another
surface have white, flat, non-angiogenic, non-healing normal serosal-synovial
surfaces. The wound margins are
obviously contracting, but they are doing what they should, following the
topological surface, meaning that instead of the defect constricting, edges
are curling under themselves. This is
completely 100% normal appropriate behavior of a healthy wound, following the
innate genetic and dynamical program that ensures that embryological
structures form and that most wounds heal properly. The problem is that it can be very frustrating
clinically to have to bear with this until the correct treatment is
done. In plastic surgery, this is a
straightforward issue of “essential coverage”, a situation that mandates some
sort of flap (or flap substitute) or else it will not “heal”. Dynamically, the situation is comparable to
grounding the circuit. The load, the
mesenchymal responder cells are simply exiting the loop, shifting away from
the stromal repair model of proliferation to the synovial metaplasia model of
maturation. As will be explained in
greater detail in Part 3, this is all part of the process of automatic
self-organization. These are the same
cells, following the same code, but they are now in a different state space,
a different “subroutine” than the wound healing space. |
|||
|
|
|
||
|
All numerical and engineering models are abstractions of the
real world. That is the purpose of
modeling, to reduce complex systems to quintessential elements that are
computationally manageable yet still accurately portray the system for the
sake of experimentation or solution.
That means that no model can be all-inclusive without reverting to the
complexity that made modeling necessary in the first place. However, if the model is well constructed,
then it is “open”, allowing components to be added or linked to account for
special circumstances. The main wound
control loop, as the “backbone” of the whole process, does indeed allow any
circumstance of disease, risk factors, and injury to be accounted for. Here are two examples. Top: This is a heel ulcer
with underlying unreconstructable arteriosclerosis. As for most heel ulcers, it healed with
topical care alone, but with retarded dynamics, taking one year to close. Ischemia suppresses or delays responder
cells which are the load in the loop. The
block labeled “circulation” is one way to show this effect. It represents arterial disease (not blood
supply). It acts as an inverting control
on the load, suppressing the load when disease is high (since the vascular
occlusion does not vary, this block could also be a switch or latch as
opposed to a continuous attenuator).
Alternatively, to more clearly portray the “dose” dependency of
arterial disease, ischemia can also be modeled as a variable impedance to act
as a retard or attenuator. Then, when
the system is normal, the impedance is bypassed and the loop operates
properly, but when ischemic, loop dynamics slow due to lower “currents” and
downstream “voltages”. Note that the
arterial disease block is an additional factor extrinsic to the core elements
of the control loop. Bottom: Pyogenic granulomas are
just excessive wound module. They form
from otherwise normal wounds. Their
name is a misnomer, since they are not really suppurative, and they are not
granulomas at all in the histo-pathological sense. They are just beads of granulation tissue,
and they almost all have one feature in common: they occur after a period of inept care,
usually poor wound hygiene covered by bandages which are unchanged and left
in place for prolonged periods, a week or more at a time – “Band-Aid disease”
if you will (with apologies to the Band-Aid company). The problem is that normal inflammatory
cells get into and function within the gauze.
The microscope picture is a specimen of an unchanged “sheer strip”
bandage, a haven for healthy active inflammatory leukocytes, including large
mononuclear cells. This has the effect
of elevating the inflammatory layer of the wound off of the surface. As in any wound, the monocyte-macrophages
issue growth factors which create a chemotaxis-chemotropism vector that draws
the wound module upward. In the case
of a pyogenic granuloma, that attraction of the wound module does not cease
at the level of the surrounding skin, but instead keeps reaching for the
source of the stimulus, the dressing.
This effect can be added to the loop as an amplified or unregulated
element parallel to normal macrophages.
As diagrammed, there is an on-of switch showing that this effect can
be started and stopped instantaneously but putting on or taking off an overrun
dressing. |
|||
|
|
|
|
|
|
This slide is an addendum to the original presentation, added to
further illustrate the multi-faceted significance of control in biological
systems and wound healing. It looks at
angiogenesis, the formation of blood vessels, and the difference between the
closed loop feedback regulation of normal embryonic angiogenesis versus the
dynamics of angiogenesis in wound healing. Normal embryonic angiogenesis, meaning the formation and
morphogenesis of a life-and-death critical vascular distribution network, is
itself a non-linear closed-loop controlled process. As such, its “laboratory” visualization
depends on modeling and computational simulation. The controlled dynamics of embryological angiogenesis
is explained by the VT (Vascular neT) model of angiogenesis, which will be
explained briefly here. Then, in order
to directly demonstrate the consequences of normal regulated embryonic
angiogenesis, photos are shown of histogenesis in a regenerative bio-matrix
(Integra® collagen-aminoglycan artificial skin). (See the Arimedica website
for extensive information about Integra collagen-gag matrix and artificial
skin. See especially http://www.arimedica.com/content/arimedica_integra
histogenesis_gottlieb-me_v2003.pdf and http://www.arimedica.com/content/ To begin, we will look at normal vasculogenesis and the dynamical controls that regulate
formation of blood vessel networks.
The VT (Vascular neT) model of angiogenesis is a numerical model of
the non-linear physiology of embryonic vasculogenesis. The model has just four inputs, a “growth
model” that governs how the host tissue grows, and three intrinsic angiogenic
parameters: “ischemia threshold” which
is how far away from the vascular network a cell in the host tissue must be
to “feel” the ischemia and trigger angiogenesis by cytokine stimulation; “reach” which governs how far from its
origin on the network to the stimulating cell a new vessel grows; “anastomosis” factor (multiple sprout
multiplier) which governs the number of new vessel sprouts which respond to
angiogenic stimulation. The iteration
of this model begins by “growing” the host tissue, meaning that the whole
domain is enlarged, then it is subdivided or “tiled” into individual cells of
uniform base size. Next, the
distance-to-network is calculated for each cell in the tissue. For any cell that is too far from the
network, it is ischemic, forcing it to generate angiogenic factors which
diffuse back and stimulate the network to sprout a new vessel. The new vessel grows toward the stimulus,
the ischemic cell, thereby relieving the conditions which triggered the
response. Obviously, this process is
iterative (recursive), non-linear (its next state depends on its current
state), and regulated by feedback (oxygen or distance to network) and closed
loop control (comparison to a reference measure of ischemia, i.e. the
numerical threshold or the bias on the vegf gene). This is how the normal biological process
works, and the numerical model simply simulates the reality with a strictly
deterministic set of rules based on the physics of diffusion and the
physiology of angiogenesis and angiogenic factors. Not surprisingly, model outputs closely
mimic real anatomy, not just vascular morphology, but also the mathematical
and engineering parameters of the network.
The model also recreates many other branched networks in a diverse
array of biological systems. Lower: This is a control diagram of
normal vasculogenesis. The feedback is oxygen levels at each cell (in the VT model, it is the
distance-to-network). The system comparator is the host or parenchymal cell which checks oxygen feedback against
the system reference (real = bias or stimulation threshold on the vegf gene; model =
ischemia threshold). Note that
feedback is inverted at the system comparator, so that when oxygen is low,
comparator output is high and there is a “green light” go on driving the
whole process. The biological error signal is the expression of angiogenic factors. The system
controller is the new vessel sprouting mechanism
(numerically and biologically). The load is the entire vascular network.
The system output is vascular density or the r-net.
In topology, an r-net is a system in which no point or element in the
space is greater than distance r from the network. Note that the new vessels are not the
system output, as you might have thought.
That would be the case if the system was trying to build a vascular
network using a vascular “blueprint” as the reference, but it is not. The new vessels change (control) the
network (the load) leading to a subsequent change in the output of the
network. The output of the network is
the unfettered availability of oxygen, which anatomically means correct
vascular density or r-values. This is
a crucial point to understand. The
blood vessels do not exist for their own sake. They are a supply network that serves the
host cells. The host, which includes
the system comparator and controller, is not explicitly trying to build a
vascular network nor does it care what the network looks like or how it is
constructed. The host only cares that
it is getting enough oxygen. Each host
cell will actively control and drive the system until its metabolic demands
are met. For these demands to be met,
every host cell must be within a certain distance of a capillary. Each capillary can service several cells
(higher or lower depending on the metabolic rate of the given tissue), and
ultimately what results is that there is a given density of vessels in the
tissue, or a given value of r (equivalent to the ischemia threshold). Growth of the host tissue is the external force which perturbs the system and diminishes vascular density and thereby
drives the formation of new vessels. Note that growth of the embedded
vascular network is strictly reactive and space driven, rather than being
autonomous and network driven. New
vessels grow only in response to needs dictated by the host cells, and this
space-regulated reactive growth leads to a controlled vascular density. It is true that nature produces a wide
variety of morphologies in vascular networks, different patterns from one
tissue to the next, one species to the next.
However, these variances form a set or family of patterns, and
vascular networks throughout nature all fall within this family. Morphological differences are determined not
by any variance in the nature of the angiogenic control loop, but solely by
the growth characteristics and metabolic demands of the host tissue. More important though is that dynamically,
vascular networks are all equivalent in that just one single process creates
them all, described by three parameters representing the diffusion and
reactions of oxygen, angiogenic cytokines, host cells, and angiocytes. Also, they are all mathematically identical
– topological fractal r-nets. Fractals
are a form of geometry in which similar morphologies repeat at different size
scales due to the repetitive application of a recursive dynamical
process. They can look complex, but
since they typically form from simple recursions with feedback and control,
those complex looks can be deceiving.
For vascular networks, the driving imperative is that no cell be
greater than some distance r from the embedded network. If you were to take a vascular network (VT
or natural), and then draw on it a simple geometric lattice (cartesian or
euclidean) using the same r-value (the way an engineer or architect might
design a distribution network), and the lengths of the two networks are
compared, the vascular network is typically no more than 10% longer than the
idealized network, sometimes less. And
since biological systems build the network only as needed, rather than
preemptively, there is even greater economy over the long run of complete
embryogenesis then adult maturation.
This economy, known in biological and other complex systems as
“parsimonious self organization” means that, for blood vessels, the body
never makes any more blood vessels than just what is needed to supply the
logistical needs of the host tissue – no more, no less – just right. Mid lower: Here are three images of
natural vessel networks juxtaposed against outputs of the VT model. On the left are meningeal vessels (the little dots demonstrate “locality”, a
crucial topological feature of vascular networks). In the middle are mesenteric and small intestinal vessels in a segment of ileum. On the right are veins in a plant leaf. Note
first the patterns or geometry of the meningeal and leaf veins. The vessel patterns are isotropic, meaning
that vessels are present equally in all directions or angles. There is no net “directionality” to the
overall field. You can rotate an image
90° and it still looks essentially the same.
This pattern results from “multiplicative” growth of the host, when
the growing host tissue is expanding uniformly throughout its entire
substance. The center image
intestinal vessels have a somewhat different morphology, anisotropic and
directional. They have long vertical
branches with acute angles which all appear to be “reaching” toward the
top. Between them are the
non-directional isotropic vessels that characterize the other images. The directional anisotropic reaching pattern
is what happens when the forming tissue has “additive” growth along a margin,
new tissue forming as a fringe at the boundary of the established tissue. This will be relevant below when talking
about wound and target angiogenesis versus embryonic and field angiogenesis. Center: This is a conceptual
graph showing the state of the wound during and after healing. It essentially shows the state of the
tissue or stroma, and the net accumulation of output from the wound control
loop (the replacement substance that restores the tissue). The horizontal dotted line represents the
target level of “stuff” that makes the tissue. First look at the brown curve. This is not wound, but rather normal
embryonic growth and development. The
tissue starts on the left with a small amount of substance. Tissue will be made, growing up to the
intended size, reaching the target dotted line at full growth. The asymptotic rise reflects that the
formation of the stroma, both the fibrous matrix and the embedded vascular
network, will grow with orderly regulated reference-controlled dynamics. The growing tissue makes only what it needs
when it needs it. Embryonic growth is
slow, but it is never excessive, and when complete, vascular density will be
precisely correct for the needs of the host cells, as expected of normal
embryonic vasculogenesis and as demonstrated by the VT model. (Collagen and matrix production have their
own controls which likewise lead to proper healthy embryonic
histo-anatomy.) Now, look at the red
curve, the wound curve. On the
left, the curve starts in negative territory, a deficiency of tissue due to
injury. Unlike for normal growth and
development, wound healing is very rapid, and the production of stromal
elements during normal wound healing overshoots the normality line. There is too much stuff, too much matrix
and vessels by the time that the wound is actually closed. In the interests of then going from the
hyperplastic scar back to normal anatomical stroma (or dermis or fascia), the
body must now start to dismantle and remodel the excesses. This process of “overshoot-then-involute”
is what the maturation phase is all about, managing the involution. Dynamically, this is equivalent to one
cycle of a damped sine, in which the system has “overcorrected” and then has
to pull back the other way. Or is it?
. . . Isn’t the purpose of control to keep everything on track without
significant variances, deficits, or excesses?
Yes. Then isn’t this overshoot contrary
to everything we have already said about wound regulation and control? No.
The explanation is in keeping all of the details and the actors and
their roles straight. Wound healing,
the Wound Main Control Loop, regulates on the state of being closed. It makes fibrous matrix and vessels as part
of system output in order to reach the condition of closed. As the wound closes, the loop is less
driven, output diminishes, and matrix and vessel production will eventually cease. The key thing is that the loop is neither
sensing nor comparing and controlling on the collagen and vessels. The loop is controlling on the state of
closed, and when that endpoint of epithelialization and closure is reached, matrix
and vessels will be whatever they are – high, low, or correct. And as it turns out, they are almost always
excessive. Once the endpoint of
closure is reached, then the hyperplastic new stroma, the scar, begins
remodeling, i.e. the maturation phase.
Top left: Two
clinical and two histological photos illustrate important aspects of
regeneration in a regenerative biomatrix.
The material shown is Integra® collagen-aminoglycan artificial
skin. When placed on a wound, platelet
and inflammatory events are arrested, and defensive responses and normal
wound healing are completely turned off.
Over a period of several weeks, new tissue forms in the spongy
matrix. Histogenesis starts when
mesenchymal stem cells wander into the sponge, then bind onto the
matrix. Once bound, they begin
proteogenesis and mitosis, forming little clusters of collagen producing
cells, cells which share all of the features of the embryonic
dermatoblast. These clusters compete
for available gases and substrate diffusing from the wound below, and when
demand outpaces supply, the clusters make vegf to attract vessels. New vessels arrive from the nearest vessels
in the tissues below, and as they arrive at the clusters, vegf turns
off. The new blood supply now allows
the dermatoblasts to go through another round of proliferation, creating the
classical fibroblasts and connective matrix that fill the voids in the sponge
and make tissue. When the process is
complete, the temporary silicone barrier layer is removed, and real
epithelium is restored with skin grafts.
The final regenerated material is histological and mechanically quite
similar to normal dermis and fascias, and quite distinct from
post-inflammatory wound healing and scar.
The dynamics and histology of the process is completely analogous to
normal embryonic mesenchymal or stromal histogenesis, and entirely unlike
normal wound healing and scar formation.
Likewise, the dynamics, geometry, and topology of the blood vessels
that regenerate in the matrix are completely analogous to embryonic
vasculogenesis, both the real process and the process abstracted in the VT
model. (See the links cited above for
more information on the very close analogy between normal dermatogenesis and
vasculogenesis and their counterparts in the regenerative matrix.) In the upper clinical picture, a bead of “granulation
tissue” – normal wound healing – pokes through a seam between pieces of the
collagen gag-matrix. Note the red
capillary-dense appearance of the normal wound versus the pale pink
appearance of the regenerated matrix.
In the lower clinical picture we see a similar situation
(different patient) after the skin grafts have been placed and healed. The healthy regenerated matrix looks
largely like normal skin, whereas the granulation tissue has become a bead of
hyperplastic scar. The upper
histology image is normal young scar in a healing wound. It is excessively dense with fibroblasts
and young collagen, without any porosity, reflecting its clinical
characteristics of thickness and hypertrophy, stiffness and non-compliance. This young scar represents the hump on the
red curve in the center panel, the peak of “overshoot”, now awaiting the
prolonged “involute” of the maturation phase which will very slowly remodel
back to a normal dermal appearance. The
lower histology image shows recently regenerated collagen-gag
matrix. Each pore or domain of the
sponge is filled with fibroblasts and collagen that have the characteristics
of normal dermis rather than scar. In
between each domain there is considerable porosity, maintaining the
mechanical properties of normal skin rather than scar. Just like in normal embryogenesis, the
regeneration of this tissue follows a convergent asymptotic trajectory
similar to the brown curve in the center panel. This trajectory is slower than wound
healing, but it never overshoots, resulting in correct histogenesis in which
stromal elements are of proper density.
The correct tissue model results because this regulated process
controls on vessel and matrix density, not on “closed” (the collagen-gag
matrix is closed to begin with because a silicone outer layer serves as a
temporary epithelial substitute). To
summarize, normal wound healing, which is referenced to the state of
“closed”, rapidly proliferates scar and overshoots correct vascular and
connective matrix density, and in so doing obliterates any semblance of
organized substructure within the scar (time frame = days). The scar must then mature, remodeling back
to the histology and mechanical characteristics of normal dermis and fascias
(time frame = months). In comparison,
the regenerative process in the collagen-gag matrix reflects normal embryonic
histogenesis. By being referenced to
vascular and collagen density, a correct model of normal tissue, with normal
dermal characteristics, is achieved by steady regulated building of the
tissue (time frame = weeks). Top middle: These two
photos are of a normal wound, wide and zoomed in, immunostained to show vegf. In the boundary between the plasma and
aminoglycan layers there are large mononuclear cells which stain heavily for
vegf. These are the system controller
monocyte-macrophages, the source of the chemotactic attraction for the
angiocytes and vessels which are streaming up from below. Review the wound histology images of slides
6-12. The initial new vessels are long
and vertical, just like the intestinal vessels on this slide. This is the effect of having a perimeter or
leading edge of attraction – a target – that is extrinsic or on the boundary of the
existing network. This is quite
different than the proper-density isotropic distribution of vessels that
exists in normal tissues, fully remodeled scar, and Integra® collagen-gag
histogenesis. Top right: Two
clinical and two histological photos illustrate the difference in vascular
density in normal wound healing versus embryonic histogenesis (as illustrated
by histogenesis in the Integra® collagen-gag matrix). The
left clinical image shows collagen-gag matrix fully
regenerated several weeks after placement.
It is seen through the silicone overlayer, awaiting final skin grafts. Note that the color of the regenerated
material is nearly identical to the surrounding normal skin, more white than
red, more collagenous than vascular.
This is because both have the same – and the proper – vascular
density. Keep in mind that both the
native skin and the regenerated matrix are alive. This might seem like an obvious trivial
statement, but it points out that both have the correct amount of circulation,
just the right number of vessels to meet the metabolic and respiratory
demands of the cells in that tissue.
In both, vasculogenesis and vascular density were determined by the
process of normal developmental angiogenesis, a controlled process that
ensures proper vascular density. The left histology image shows the regenerated collagen-gag matrix. Blood vessels are few, with a density
comparable to normal dermis and fascias, and a density which is precisely
correct for the needs of the tissue. Vessel
caliber is small and uniform, typical of normal capillaries and pre- and
post-capillaries. In comparison, the right clinical image shows “granulation tissue” in a normal wound. Usually taken as a sign of a healthy wound
and wound healing competency, this red pebbled appearance represents the
vessels that have formed in the mid strata of the wound. Seen in comparison to the paleness of
normal tissues, the saturated red color is indicative of significant vascular
hyperplasia and increased vascular density.
The right histology image shows granulation tissue from a
wound similar to the clinical photo.
It shows organized vessels in the histio-attraction layer at the
transition between the aminoglycan and fibrous zones. It is shown at roughly the same scale as
the left histology image of regenerated matrix. The angiogenesis of normal post-inflammatory
wound healing is seen as dense,
closely distributed large vessels carrying large volumes of blood. This high blood density is why granulation
tissue is seen grossly as an exuberant saturated red color. The collagen-gag matrix undergoes a
histogenetic process that is comparable to normal embryogenesis. When looking at normal tissues (dermis,
fascia, regenerated matrix) they appear pale.
They also have normal vascular density, the correct vascular density
to meet their metabolic needs. This is
because the non-linear regulatory mechanism that controls angiogenic
embryology controls on vascular density, striving to make precisely the
correct number of vessels needed to supply the host cells. Thus, these tissues appear pale because
normal vascular density is what it is, and that is the way the tissues look
at proper density. This is the color
of the correct circulation to meet the metabolic needs of the host. In comparison, the bright red “granulation
tissue” in a healthy wound has excessive vascular density. However, the metabolic requirements of the
wound are not extraordinarily different than those of normal skin and
fascia. The vascular density of the
granulation tissue is thus excessive, exceeding the metabolic requirements of the
host tissue. If embryonic angiogenesis
is a reference-regulated control process, and if wound healing is likewise a
reference-regulated control process, then why is there such a difference in
vascular density? We have just looked at a potpourri of
different items on this one slide.
What is the common thread?
Simple. We can now explain why
the vascular (and collagen) density in normal embryonic tissues and in
regenerative matrices is low (normal) versus why it is so high in
post-inflammatory wound healing. It
all comes down to basic elements of control – what are the system reference,
feedback, and comparator, and what are the controller, load, and output? What are they (which cellular and chemical
elements of the system), and also where are they, in which strata or locales? (Let us first reiterate that normal
embryonic histogenesis and regeneration in the collagen-gag matrix are
essentially the same thing. They have
the same basic dynamics and anatomical organization. The explicit details of their similarities
are beyond the scope of this presentation, but can be reviewed at the links
given above. For the sake of this
slide, the collagen-gag matrix was used as a simple and readily available way
to observe these dynamics, in lieu of actual embryological specimens. Below, when embryology or matrigenesis are
mentioned individually, generally each implies the other as a type of “normal
histogenesis”.) In normal
histogenesis, the system reference and regulatory
controls are all about the matrix itself, the density of vessels and
collagen. Angiogenesis per se is
controlled. (This is easy to
demonstrate and understand via the VT model of vasculogenesis.) The feedback is a function of the vessels,
their density, and the oxygen they deliver.
The system reference is the amount of oxygen expected by the vegf
gene. The system comparator or error
detector are the developing local parenchymal or histiogenic cells which are
trying to form the tissue.
Angiogenesis is not triggered until “native” cell mass and metabolic
load in scattered independent locales exceed the existing blood supply. When need does exceed supply, when new host
cells need their own vessels, then they summon the formation of new vessels
via the error signal of angiogenic factors.
This stimulates the controller, the vessel sprouting mechanism, and
new vessels are sent directly to the locales where beckoned. The load, the actual vascular network, is
thereby modified, and the output as perceived by the host is the density of
the network which governs the oxygen feedback parameters. As new vessels arrive and the blood supply needs
of the host cells are satisfied, then the angiogenic cytokine error signal
turns off, and there is no further stimulus on the vessels. The feedback and regulation, i.e. the
closed loop control, are thus between the growing embryonic host cells and
the blood vessels they attract. It is
the vessels themselves that are regulated, leading to a defined vascular
density. Turning angiogenic
stimulation on only when vessels are needed, and then turning off, means that
only just the necessary number of new vessels appears, and the host tissue has
precisely the right density of blood vessels needed for normal development
and function. One clinical consequence
of this is that normal tissues appear “pale”, desaturated of red color
because the required vessel density and blood volume in normal tissues is
only a slight fraction of net tissue mass. In post-inflammatory
wound healing, matrigenesis is the system output,
but regulatory control is not about the matrix itself. Regulatory control is between
platelet-plasma-leukocyte error detectors and the state of open-versus-closed,
and the state of closed does not exist until epithelium fully sequesters
mesenchyme. It is also true that
epithelium cannot grow and close unless the underlying matrix is properly
proliferated. The output of
collagen-vascular stromal matrix is thus make-or-break essential to the
system, but nonetheless it is not the controlled parameter. If we focus on the interaction between stimulatory
macrophages (the system controller) and the responder angiocytes (the
controlled load), the process is one way only, a strictly open loop direct relationship
between these two events. Macrophages
trigger the creation of new vessels, but revascularization does not suppress
the macrophages. When new vessels
arrive at the controlling macrophages, the macrophages just keep on pumping
out angiogenic cytokines and triggering more vessels. This is because the macrophages are being
transformed and stimulated by inflammation, not ischemia. Inflammation and the error detectors neither
know nor care what vessel and matrix density are. The feedback that works its way clockwise
through the control loop and eventually back to the macrophages must do so
through several other intermediary control blocks, creating time delays or
phase lags. By the time the wound
starts closing and macrophages are down-regulated, the output of vessels and
matrix will be whatever they are, and they are dense, far in excess of what
is normally needed by healthy tissues.
The clinical and histological appearance is of excessive numbers of unnecessarily
large blood vessels, a substantial percentage of wound volume (in the
relevant strata), forming bright red “granulation tissue”. With regard to angiogenesis and
vascular density, here are the important contrasts between normal and wound
healing histogenesis. (1) In normal histogenesis, host cells (comparator,
the system master) sense inadequate vessels then attract new ones. Restored vessels then inhibit the
attraction – a straightforward system of simple closed loop control. In wound healing, the macrophages are the
controller (only a system agent) which attract vessels, and being regulated
by inflammation they are completely indifferent to the state of the
vessels. The arrival and restoration
of vessels does not inhibit the attraction – an open loop unregulated system. (2) In normal histogenesis, the
host cells which attract vessels are an intrinsic part of the developing
tissue. The host cells and the vessels
they attract will have a lifelong fixed relationship to each other, so it is
crucial that they have a mutually regulated interaction to build proper
tissue architecture. In wound
healing, the cells which force and attract vessels, the macrophages, are
transient players extrinsic to the regenerating stroma. They have no other relationship to the new
vessels, and they clear the scene after they have served their purpose. (3) In normal histogenesis, vessels
and density are the measure of the system that is compared to the
reference. Vessel growth and density
are thus explicitly and precisely controlled, accurate and efficient, neither
more nor less than what is required by the host. In wound healing, vessels-density-oxygen
are not the measure of the system.
Vessel growth and density are unknowingly overdriven, overshooting
normal values by the time the wound is closed, resulting in the excessive
vascular density seen as “granulation tissue”. (4) In normal development, the
developing host cells are dispersed or distributed evenly throughout the
histogenetic field. This results in
“field angiogenesis”, and the resulting vascular patterns are isotropic with
no overall directionality, exhibiting the orderly topological feature of
“locality”. In wound healing, the
macrophage and angio-attraction zone is a layer near the top of the wound. This results in “target angiogenesis”, with
all new vessels attracted along the same spatial vector, from below to above,
creating an anisotropic directionality to the new vessels. (5) Developmental angiogenesis, in
which vessels and correct vascular density are explicitly controlled, include
conditions of embryonic and maturational growth and development, and also embryonic
type neo-histogenesis such as in Integra® collagen-gag matrix and in
acellular cadaveric dermal matrices. Non-regulated
excess density angiogenesis occurs from incidental conditions of trauma,
pathology, and targeted stimulus, such as wounds, tumors, placenta, and
certain implants. |
|
||
|
|
|
||
|
Most cursory or perfunctory discussions of wounds focus on a few
major categories of causative or associated
diagnosis – arterial, diabetes, pressure, etc. What is ignored is that there are also
diseases of the wound healing system.
The whole premise of this series of lectures is to elucidate what are
the core intrinsic diseases of wound healing.
However, unlike the way we think about most diseases, the intrinsic
wound diseases are not due to a single gene mutation, not due to an excess or
deficiency of a specific metabolite, not due to dysplasia or degeneration of
any specific cell or tissue. Simple
biochemistry, proteomics, and genomics cannot explain wound failure and wound
healing incompetence. Understanding
the intrinsic pathologies of wound healing depends on understanding the wound
as a physical system. The relevant
science is physics. Understanding wound physics begins with understanding that the wound,
like most complex biological processes and structures, is a non-linear
controlled system. We have just
described the main control loop of the wound as the core of this controlled
reactive system. The loop explains how
the body responds to perturbations of the wound and the wound healing process.
When wound disorders or pathologies
occur, they can be of two types with reference to the intrinsic loop. They might be (1) aberrant behavior of the
control loop due to diseases or derangements that sicken or impair its
elements, or (2) aberrant outputs or loop dynamics due to proper reaction of
the loop to aberrant or extreme inputs (extrinsic stresses) which perturb the
system. As will be discussed in detail
in Part 3 of these lectures, there are not many diseases of the loop
elements, i.e. conventional biological diseases of macrophages or angiocytes
and the like. More often, wound
failure and incompetence is due to the types of stresses, perturbations,
forces, inputs that challenge the system.
The main question to ask is, what is the difference between healthy
wounds that heal easily, and chronic and pathological wounds that do not heal
easily? The answers to this question
are the purpose of these lectures, and they are forthcoming. A definition is needed here: the CAP wound – Chronic and Pathological. Chronic and pathological CAP wounds are those
that are either (1) caused by a chronic illness or pathology, or (2) fail due
to disease or incompetence of the healing process. Some cap wounds are due to identifiable
extrinsic diseases, such as arterial problems or repetitive trauma. They are the ones that are usually easier
to heal, as long as the primary disease is resolved. Cap wounds that are due to inherent wound
healing problems – i.e. wound healing is “broken” – are the ones that are
difficult to heal. |
|||
|
|
|
||
|
So far we have explored the issues of closed loop feedback,
non-linearity, and control, and how those principles underlie the dynamical
timewise physiology of the normal wound, and even the stressed or impaired
wound. What does all of this have to
do with the sick wound and the CAP wound, chronic and pathological? CAP wounds are often hard to heal and refractory to care. Dynamically, the control loop and the
behavior of the wound are non-convergent, i.e. they are divergent or chaotic.
The reasons why are found in the study
of non-linearity, complexity, and complex systems. These reasons will be explored in detail in
Part 3. As a prelude, consider that
these faulty dynamics mostly occur when pathological stressors are present in
a wound, forcing the Main Control Loop to get locked into complex states that
prevent resolution of the wound. The
relevant concepts that explain why this happens, subtopics of non-linear
dynamics, include: control & multi-control, N-body dynamics & chaos, population
logistics, and cellular automata & self-organization. The graphics are mathematical items from non-linear dynamics
that illustrate these concepts. They
are: (top) logistical maps and mandelbrot sets illustrate non-linearity and
population dynamics; (middle) pumps illustrate N-body dynamics and
multi-control; (bottom) mandelbrot sets are matched to corresponding wound
dynamics. In Part 3, it will be shown
in detail how the dynamical properties of complex systems, as illustrated by
these objects, interact with anatomical and pathological alterations in a
wound to create chronicity. The
following 2 slides are a preliminary introduction to one crucial component of
these dynamics – the concept and origins of chaos in the wound. |
|||
|
|
|
||
|
Control is control, and the principles and physics do not
vary. Yet different systems can appear
to behave very differently due to the nature of their elements, their
reference, and the nature of the perturbations or stresses on the
system. Go back to the endocrine and
wound comparison of slides 18 & 19.
The hypothalamic-pituitary-endocrine control system is a free-running,
continuously monitored loop. There is
a constant level of thyroid hormone output.
If it varies, the system will respond to restore the required level. There are diurnal periodicities and
incidental reactions to various stresses, but at all times there is a basal system
output that must be monitored and regulated.
The sustained basal rate of hormone production might be due to an
autonomous pacer or load controller in one of the glands, versus the basal
level just reflecting control around the system reference, whatever that might
be. Either way, control dynamics are
what they are for all systems, the same generic principles reacting to the different
physical realities of each particular machine. In comparison to the endocrine system, the Wound Main Control
Loop is an ad hoc reserve system on quiet standby, asleep if you will, reacting
if and only if and only when it is needed in response to incidental triggers. On slide 23 we showed that there are some
standard ways in which controlled systems might respond to perturbation. These responses depend on the intrinsic
quality and tuning of the machine, and then on the nature – strength,
profile, duration – of the perturbations.
As we first saw on slide 26, that for the wound and for many other
biological systems, three responses are likely to occur: convergence,
divergence, chaos. For the endocrine
control system, convergence is on the basal level of hormone production,
which is a continuous positive outflow of hormone. For the wound system, convergence is on a
healed wound and repaired stroma, a zero in the system, after which the
system goes quiet, in hibernation until it hears the next knock on the door
of injury. Divergence is an
undesirable morbid state in which control is overwhelmed by strong inputs
versus illness within the loop which keeps it from reacting properly. Chaos reflects complexity within the
system, either its natural state, or its state under the influence of
stressors. The three likely dynamical states – attractors – of the wound
control system each reflect certain clinical and pathophysiological states of
the wound. (1) The healthy wound
is healing properly and closing, dynamically convergent. (2) The sick wound is actively
pathological, ulcerating and enlarging, dynamically divergent. (3) The impaired wound is stagnant,
“orbiting”, getting neither better nor worse, dynamically “chaotic”. The disorders and diseases of the wound
healing process all obviously have a tangible physical reality based on
chemistry and biology, but how those physical elements inter-operate to get
the job done, or fail, is a matter of physics. Looking for the reasons why wounds do not
heal will not be resolved by discovering the combative chromosome or the evil
enzyme or the lazy leukocyte. Every
biochemical and biological discovery made adds another vital piece to the
whole puzzle, but the rules of how to assemble and solve the puzzle are
dynamical and operational – physics.
As will be detailed in Part 3, the chaotic non-healing impaired wound
actually has nothing really wrong with any of its constituent parts. It is only their integrated operations that
have gone awry, because of the reactions of the wound control system to the
circumstances of the moment. |
|||
|
|
|
||
|
The dynamics of the impaired wound will be discussed in great
detail in Part 3. As an introduction
though, we will look here at some of the dynamical events that shift the
wound control loop from one of convergence and proper healing to one of
disordered dynamics and failure to close.
This starts by understanding that dynamically, normal unimpaired wound
healing is a convergent one-shot. The
aggregate response is a one-shot, and the response of each of its constituent
subsystems is a linear series of one-shots.
The dynamic of the impaired wound – chaos – begins when these orderly
convergent one-shots are disturbed, either by extrinsic stresses on the
system or by illness within the system. In the upper panel, time is represented by a logarithmic
scale. The curves shown are idealized
abstractions of the wound and its dynamics.
This is the conventional linear-sequential concept of wound healing,
and it is essentially equivalent and mappable to the closed loop control
model for a particular subset of wound dynamics, the
healthy-healing-convergent wound. For
a moment, just focus on the “wound module” curve. This is a non-rigorous conceptual curve. As the curve goes high and then low, it
represents the load and output of the wound repair cells, their kinetics as
driven by the wound control loop, ramping up as they become activated, then
decaying back to standby as they wrap up business as the wound closes. Next, note that this conceptual kinetic
curve or curve-of-state is repeated for different main events: thrombosis,
inflammation, wound module, and maturation.
All of these events are related to each other, but each is also its
own control system. Recall from the
Main Wound Control Loop that inflammation, the error signal, is a black box
element, but within that black box is a highly complex system that “knows”
how to turn on and turn off and do its business in response to injury,
disease, and the blood-based error detector.
Consider the roles and dynamics of each of these major events in the
response to injury: Injury: Consider what happens
when a one-time incidental self-limited injury occurs – a cut finger, a
surgical incision, a ruptured tendon, whatever. It occurs in the time frame of seconds-to-minutes,
and then it is over. It too will have
the same general shape as the other curves shown, and it could / should be
added to the diagram as another hump on the left side, the “injury” cusp. The effect of the injury is to trigger
thrombosis. The injury itself was
brief, and by the time that thrombosis is really getting underway, the injury
itself is over. Thrombosis: The term
“thrombosis” is used here in a loose sense to refer to the aggregate injury
recognition events that are based on a variety of blood borne elements – mainly
plasma proteins, platelets, and leukocytes.
In the historically conventional sequential-linear view of wound
healing, thrombosis is the on-off switch which recognizes injury and gets the
rest of the process going. In the
non-linear control view of the wound, thrombosis is the error detector which
is comparing current status to normal tissues and issuing an error signal in
the form of inflammation. (These two
views are essentially the same thing when injury and response are simple
healthy one-shots, but they diverge when wound dynamics become chaotic, which
is why a proper non-linear model is best suited for non-linear real events.) In the wound control loop, “thrombosis” or
the error detector is a black box, but unto itself within that black box is another
complex control system. When
“thrombosis” is triggered, there are two factors which cause thrombosis to
“rise” or ramp up: (1) the more potent
and the more sustained the injury or trigger is (the integral of the trigger
with respect to time), then the more potent is the net response; (2) thrombosis is also an auto-amplifying
event which raises its own kinetics.
Thrombosis will eventually reach a peak, and then start to decay. If injury was a one-time limited event, a
one-shot, then there is nothing further to trigger thrombosis beyond the
initial event. Thus, thrombosis gets its
one-shot trigger, then it runs its course, and then it decays and
extinguishes. (The term “one-shot”
comes from electronics, jargon for a “monostable vibrator”, a circuit which
can switch state (on-off, positive-negative) when triggered, and then it
returns to baseline by discharge through an R-C circuit. The basis for many timing circuits, the
output waveform and the time-dependent nature of the electronic event is
completely analogous to the phases of injury-response as shown here.) Aside from its immediate effect to staunch
bleeding, thrombosis has a crucial dynamical effect – it feeds forward into
the control loop by triggering inflammation.
Thrombosis events build then decay in the time frame of
minutes-to-hours. By the time that
inflammation is building, acute thrombosis events have largely subsided
(there will still be old fibrin thrombi and platelet plugs in the field, but
no new platelet aggregation, fibrin catalysis, nor clot formation is occurring). Inflammation: In the
historically conventional sequential-linear view of wound healing,
inflammation is the next downstream event after thrombosis and the trigger
for subsequent wound repair. In the
non-linear control view of the wound, inflammation is the error signal which
activates the system controller.
(Again, these two views coincide for single event “healthy’ injury and
one-shot responses, but they diverge when wound dynamics become
chaotic.) Inflammation is turned on by
an integrator effect of thrombosis.
Thrombosis events build or accumulate the stimuli needed to trigger
inflammation, and once inflammation begins, it can be self-sustaining, even
as active thrombosis wanes. In the
wound control loop, inflammation is abstracted to a single element, the
control signal, but within its own black box it is its own complex
multicontrol system. It too is
self-amplifying, so between the thrombosis trigger then its own dynamics, it
builds to a peak effect. Assuming that
injury and thrombosis were one-time one-shot events, then there is no further
provocation for inflammation, and inflammation events will soon enough start
to decay and wane. Aside from its important
short term effects to defend the host and clean up the injury, inflammation
also has the crucial dynamical effect of feeding forward into the control
loop by spawning and regulating the system controller, the macrophage. In the same way that thrombosis triggers
inflammation, inflammation is itself an integrator function that gradually
builds the controller in such a way that the controller can be
self-sustaining as inflammation subsides.
Inflammation events build then decay in the time frame of hours-to-days. By the time that the macrophage population
is built and load control and system output begin, acute inflammation events
have largely subsided. Repair: Note the dynamical
similarities between the injury-thrombosis-inflammation cusps, and the
analogous transitions from one phase to another. That trend now continues in the jump from
inflammation to the repair phase, the proliferative wound module. Once triggered, repair events will build to
some peak of productive activity. As
system output increases and thus the wound diminishes, the control loop is
less driven, and wound module kinetics start to wind down. Wound module ramps up then decays in the
same way that the preceding phases did, but in a time frame of
days-to-weeks. Wound module is also an
integrator function that triggers another downstream one-shot event – maturation.
Maturation begins only once the wound is epithelialized, meaning that
maturation waxes only as wound module wanes. Maturation: The
integrated output of the wound control loop, i.e. the net production of the
output control block is the reconstituted stroma that negates the condition
of “open wound”. However, it is not
normal stroma, it is scar, a form of stroma that is excessively dense with
vessels and connective matrix. This
undergoes a process of remodeling or maturation that eventually restores it
to proper architecture and density of the stromal structures. The way wound healing and the wound control
loop have been defined here, the wound is healed and the loop settles when
epithelialization and closure are complete.
Maturation is therefore a tangential or derivative event, and like
everything else it is its own control system within its own black box
domain. The scar is the integrated
output of the wound module, but also the feed forward input into the
maturation block and curve. As for the
other major events, maturation rises to a peak dynamic then fades, with its
time frame measured in weeks-to-months. In the dynamics of the normal wound, in the progression of
injury à thrombosis à inflammation à repair à maturation,
there is a crucial additional relationship implicit in the diagram – the anatomical
stratification and timewise separation of these events. Yes, these events are contingent, each
downstream event being triggered by the preceding cusp. However, aside from a handshake and the
flip of a switch to get things going, these events and phases, and their
constituent cells and structures, actually have only limited contact with
each other. Recall from the review of
basic wound anatomy that the wound is not just an amorphous bowl of
pudding. It is highly structured, even
within the few vertical microns or millimeters of its existence. The vertical anatomy of the wound is a
timewise historical view of what has happened after injury, with the top
happening now, and the strata underneath being progressively older. Thrombosis events are in the topmost
layer. Inflammation is there and in
the aminoglycan layer underneath, repair events are below that, and
maturation occurs later (more on this in Part 2, see especially slide
52). These events overlap to some
extent, vertically and in time, but the overlap is comparable to the overlap
of cusps in the sequential phase diagram shown here, with preceding events
waning as latter phases appear. As
long as these phase relationships are maintained, and as long as there is no
new injury, then each event runs its course with one-shot dynamics, and the
net dynamics of the wound runs a smooth course from injury to maturation. In the lower panel, we can start to see where chaotic
dynamics come from. This diagram
likewise is missing the acute injury cusp at the left. However, imagine it is there, and consider
this scenario. A single injury occurs,
putting the other events into action, each following the other, each having
one-shot dynamics, and each conforming to the general shape of the cusps
shown. Keeping an eye on the
logarithmic timescale at the bottom, imagine that at 12 - 24 hours another
injury event occurs. Within a few
hours, the downslope of the inflammation cusp will suddenly have another
rise. This will result in new uprises
in the other downstream events. Each
event curve will then seem a bit more complex, although obviously the new
blips represent the superposition of the new curves on the original
ones. If more injury events were to
occur, then more event curves or phase cusps would have to be added. Assuming that each injury event leads to an
exact repeatable profile of downstream events, then the resulting composite
activity curves would be the superposition of x-number of original events,
and the curves might start to seem very erratic or complex. Understand the significance of the phrase
“exact repeatable profile of events”.
This means that the system response adheres to a precise set of rules
– i.e. it is strictly deterministic. Although
the composite activity profiles might seem complex, they just represent the
superposition of multiple copies of the same “waveform”. If you knew the precise shape or dynamics
of a single response curve, then the entire data stream could be
“deconvoluted” (a mathematical method) to see all of the individual injury
events and the time that they initiated.
The point is that with repetitive injury or events, the composite data
stream might seem very erratic or “non-functional” or “non-analytical”
(unlike a simple equation or standard function), yet there is strict
rhyme-and-reason to the data. The real situation in real wounds actually gets even more
complex, but the principles of deterministic rules and the superposition of
waveforms remain in force. In the
lower panel, note the double arrows between the thrombosis, inflammation, and
wound module cusps. In the normal
healthy unstressed response to injury (the normal wound control loop), all of
the dependencies between one control block and another feed forward, and the
control loop runs clockwise. The net
effect is the smooth one-shot convergent dynamics of normal healing. These double arrows, back and forth, imply
a retrograde stimulus or feedback. How
is that possible? It is possible, it
does happen, and it is abnormal.
“Abnormal” in the words of medicine is “pathology”. These retrograde, events are the
consequence of sustained injury or diseased and disordered states of the
wound. Consider first the
inter-relationship between thrombosis and inflammation. This is discussed at length in Parts 2
& 3 (e.g. slides 2-46 & 3-07).
First, thrombosis triggers inflammation. From the point of view of conventional
biology, thrombosis is explicitly how injury is recognized which then turns
on inflammation. Dynamically, this is
the normal feed-forward relationship of injury-recognition (system
comparator) to injury-response (error signal), with one-shot thrombosis
waning as one-shot inflammation is rising.
However, inflammation can also cause thrombosis. Inflammation creates a variety of chemical
mediators, mechanical-geometric-flow changes in vessels, changes in blood
rheology, activation of leukocytes, and even further stasis and activation of
platelets, all of which are potent thrombogens. The retrograde trigger of thrombosis by
inflammation occurs under special circumstances, circumstances in which acute
inflammation is sustained by some condition of continued injury or disease. When inflammation is sustained beyond its
ideal one-shot profile, then it begins to act as an integrator or accumulator
of those factors which risk triggering thrombosis. Inflammation can also cause another “retrograde” dynamic – creating
more injury. This was explained on
slide 24. This is an effect of the
destructive histiolytic components of inflammation. These lytic effects are normal, meant to
destroy exogenous pathogens if present and especially to dissolve damaged
stroma so that phagocytic and repair cells can perform their tasks. Normally, these lytic effects act on the
original injury and damaged tissue. By
the time that wound module and repair are ramping up, inflammation is waning,
and inflammation will not have destructive effects on the re-forming
stroma. However, if some condition of
repetitive injury, thrombosis, or other triggers of inflammation re-activate
or even perpetuate acute inflammation, then inflammation will become fully
active concurrent with active repair.
Under those conditions, the destructive effects of inflammation not
only suppress repair, but risk creating damage and destruction of restored or
surrounding tissues, thereby causing progressive ulceration. Consider too that thrombosis can have a retrograde
effect to cause injury. Normally,
thrombosis is a response to injury and damaged tissues. By the time that normal first-round
inflammation and then repair are active, thrombosis is long gone, and it has
no effect on subsequent new tissues.
However, if some act of recurrent injury or sustained inflammation is
present, enough to cause thrombosis when it should be gone, when things are
trying to repair, then what happens?
Thrombosis obstructs blood flow and causes infarcts. In chronic wounds, wound infarcts can and
do occur. Small and multifocal, the
aggregate effect can be quite damaging to the wound as a whole, undoing what
has already been built, or causing
progressive ulceration.
Infarcted granulation tissue is a common observation in chronic
wounds, such as coagulopathic and immunopathic wounds when there is a flareup
of active disease. Next, note the
wound module cusp on the lower panel.
It never rises to full activity because of suppressive effects of
sustained inflammation. These abnormal
inter-phase dependencies can feed forward and be inhibitory as well as feed
backward and be stimulatory. Finally, the
lower panel also shows a backward arrow from repair to inflammation, another
abnormal retrograde event that is not a normal property of healthy clockwise
wound healing. This abnormal
dependency is explained in detail in Parts 2 & 3. The effect of new injury events, new thrombosis events, and all
of these counterclockwise retrograde events is that if one occurs, then a
preceding phase is bumped up to a new wave of response and reaction. This new bump then feeds forward,
clockwise, sustaining overall wound dynamics.
What should have been an up-and-down one-shot event then becomes
disordered as the “waveforms” of each of these new rounds of activity are
superimposed. The immediate response
and dependency of one phase on another remains strictly deterministic,
strictly driven by the physical, chemical, and biological rules of the
system. However, by the time that
enough of these aberrations have occurred, the waveform or data stream of
sustained unsettled loop activity can seem anything but orderly. Instead of each phase or event rising and
falling in sequence, eventually all events are simultaneously sustained and
active. This is seen on the right side
of the lower panel, where each event has a continued but erratic profile that
continues without termination as long as the chronic wound persists. If you were to record some parameter of all of this – wound
size, loop dynamics, an inflammatory chemical concentration, density of a
certain cell, whatever - what would
you find? We have just seen that
things might seem erratic, but we also know that the responses are strictly
rule based and deterministic. If the
responses or transfer functions between phases or events are standard,
understandable, and analytical, and if all of the superimposed states or
values of the system can be convoluted or deconvoluted, then shouldn’t we be
able to parse or decode the data stream into its constituent events? If the cusps have a periodic or standard
time-delayed relationship to each other, then shouldn’t we be able to find
some evidence of periodicity or harmonics in the data? Or is the erratic data so disordered that
there is no order, that instead it is just noise, i.e. random? No, no, and no. No, you will not be able to decode or
deconvolute the data stream to a set of original simple or primary waveforms
(unless you know with infinite precision every detail of the system, aka
LaPlace’s Demon). No, neither a
fast-inverse fourier transform nor other relevant method is likely to have a
decipherable power spectrum, i.e. no frequencies or periodicities in the
data. And surprisingly, the data is
not likely to have a uniform or gaussian distribution to imply strict
randomness. If you know how to map or
graph the data properly, there will be structure in the data (explained in
Part 3). What is that structure? Chaos.
The nature of deterministic chaos was explained on slide 23, and now on
the right side of the lower panel on this slide you can see what it looks
like. Remember, “chaos” in
math-physics-engineering isn’t the same as vernacular chaos. It is strictly rule based and
non-random. It can be highly complex
and at first seemingly undecipherable, but it is not arbitrary. Everything you see in the data stream has a
mappable cause-and-effect relationship to something else or even to its own
antecedent or resulting state. It
looks wild and crazy and unpredictable, but it is the result of highly deterministic
analytical functions which become entangled or superimposed or have abnormal
feedbacks which retrigger or amplify the system in complex patterns. We have been discussing that wound and wound loop dynamics will
tend to fit three profiles of response: one-shot convergence,
saturation-divergence, or chaos. You
can see here what chaos really means to the system, and why it happens. In the wound, it happens when the orderly
progression of wound phases and the orderly sequencing of control block dynamics
as a set of single-cusp one-shots gets disordered. The disorder results from repetitive
primary injury extrinsic to the loop, and from abnormal intrinsic feedbacks
and retriggers. These abnormal or
unanticipated events and dependencies have an effect to sustain the wound and
loop dynamics. This sustentation can
eventually reach a point where the repetitive or persistent interplay and
recurrence of injury-thrombosis-inflammation can become a self-sustaining
state that can no longer find a path to convergence. Since healthy wounds heal properly, what
types of disease can induce a wound to get locked into a chaotic “attractor”
of this sort? Keeping in mind the
circular dependencies of thrombosis and inflammation, and the effects of both
to create more injury, the diseases that induce chaotic wound dynamics are
sustained conditions of primary injury, primary thrombosis, and primary
inflammation. These include repetitive
trauma, autoimmune states, atopy-allergy disorders, chronic infections, and
hypercoagulable and micro-occlusive disorders of blood or blood vessels. Parts 2 & 3 of this series will explain
this in detail. |
|||
|
|
|
||
|
This slide is a reminder that this is a three part presentation
that looks at wound pathology from the point of view of its applicable
physics, elucidating the intrinsic dysfunctions of the wound as a result of
dysdynamia, especially when stromal auto-immunization has occurred due to
prolonged population admixture in a repetitively injured wound. Part 1 – The Wound as a System and a Controlled Machine The wound module, the wound control loop, wound pathology, and the basic dynamics of healthy and impaired wounds. Part 2 – Auto-Immunopathy and the Intrinsic Disease of Wound
Healing The cellular & histopathological basis of intrinsic wound
failure & wound chronicity: chronic inflammation, wound autoimmunopathy, &
the 3-population wound. Part 3 – Chronicity and the Physics of
Wound Failure The physics of wound failure and chronicity: N-body dynamics and chaos, population logistics,
cellular automata and self-organization. These presentations and supplementary
materials are all available at www.arimedica.com . |
|||
|
|
|
||
|
We have now concluded the first part of this series on The
Physics and Pathology of Wounds, looking at wound healing as a system and a controlled
machine. As for any complex system,
biological or non-biological, it is the presence of non-linear feedback and
control which governs the basic dynamics and timewise behavior of normal
wound healing. As a complex non-linear
controlled machine, the self-organizing wound must be studied as such (and not
just as a collection of isolated one-on-one elements) in order to fully
appreciate both its normal healthy dynamics and also its response to
perturbation and pathology. The
complex non-linear “machine” that is wound healing can be diagrammed as a
foundation closed-loop control system, the Main Wound Control Loop. Understanding these dynamics will be
essential in Part 3 of this series where we will look at how and why wound
dynamics misbehave and wounds fail to heal.
The following comments summarize the key points. The wound is a control system.
It is a reactive reference-driven feedback system that ensures the
correct result – the restoration of the mesenchymal stroma and its
sequestration by a regenerated epithelium.
Control also makes sure that the wound healing system stays asleep
until needed, then comes to life, builds a morphologically correct structure
with just a few rules and cell types, then winds down and reenters standby
mode once its job is complete. As a
complex controlled system, no amount of conventional bioscience
experimentation will elucidate the operational physiology of the impaired wound. Required are the relevant tools of physics
and engineering – non-linear dynamics.
The wound module is a control loop. The wound healing system is a small set of
key cells and interactions, each with a designated time, place, and function
within the anatomical wound. They also
have their place on a basic engineering control loop that has feedback and a
way to correct variances from a reference state. The Main Wound Control Loop simply explains
how the kernel elements of repair interact to correct the effects of injury. The healthy wound and all states of pathology
can be modeled on this loop. The wound is dynamical, it has timewise behaviors: Healthy acute wounds heal as a one-shot
convergence back to reference (reorganized & closed). Actively pathological and ulcerating wounds
are sick, in a state of saturated divergence, getting
progressively worse and with a loss of control. Chronic and pathological CAP wounds are the
impaired wounds, chaotic, orbiting on attractors that are
dynamically hard to change. Modeling wounds:
The wound control loop is an open kernel. Dynamical behaviors, healthy and altered,
can all be studied or understood from this perspective. Physiologic, pathologic, and therapeutic
elements, and also their interactions, states, and events can all be modeled
into the loop to show where errors occur and where therapy can be introduced. In human engineered and technological
systems, machines are designed to have a particular stability or output. Good control is based on understanding how
a signal or state must be transformed through a control block (transfer
function), and it is important to design a system with correct feedback and
responses to avoid oscillations or instabilities. In support of these goals, there are robust
math and engineering methods, such as second order differentials,
convolutions, and LaPlace transforms, which permit the exact design and
precise numerical specification of a planned machine and its operations and
output. In principle, precise
disciplined engineering could apply as well to the wound . . . in principle. However, we have too little knowledge of
wound physics to have precise engineering specs and numerical
characterizations of the wound machine.
However, until complexity and the science of systems is acknowledged
for wounds, and the relevant 21st century tools and means of
research are put into practice, there is a risk that we will never elucidate
thoroughly dependable therapies. We
risk never getting out of our current method of haphazard, hit-or-miss,
try-this-try-that management for the hard-to-heal impaired chaotic wound.
For the chronic and
pathological CAP wound, where the road to the desired result is not so easily
traveled, and for which conventional 20th century bioscience has
yet to even reveal a failsafe road, understanding systems level behavior and
failure is long overdue. |
|||
|
|
|
||
|
End |
|||
|
|
|
||
|
Abstract (as submitted in advance of the meeting) The Physics and Pathology of
Wounds. Part 1. The Wound as a System and a Controlled
Machine. Marc E. Gottlieb, MD, FACS The wound is a transient organ of
inter-operating cells, triggered into being by injury and inflammation, then
extinguishing as it completes its repair of injured stroma. It is a system.
Conventional bioscience tends to characterize properties and interactions of individual
or one-versus-another elements within a system, but physics is required to
understand the integrated timewise behavior of whole systems. Intrinsic wound
pathology and chronicity, and wound failure and therapeutics are easily
explained when wounds are seen as a non-linear System (rather than as a
collection of dual-element linear interactions). For normal wound physiology
and for the pathophysiology of altered and failing wounds, the governing
principles are the physics of complex systems: non-linear N-element dynamics,
control science, population logistics, and self-organizing automata. Understanding wound physics begins by
characterizing normal wound physiology. The wound is a closed-loop
reference-driven non-linear multicontrol system. Sick and altered wounds have
layers of added complexity, but the quintessential intrinsic machinery of
wound healing – the Wound Module of post-inflammatory wound repair –
functions as just a single control loop. When tissues are injured, the Main
Control Loop of physiological wound repair will drive cells to reorganize
back to a repaired stroma. The wound control system is composed
of these elements: The system state is the open wound and its conditions. It
is compared (?) to a reference, normal epithelialized tissue. Variances
generate an error signal in the form of inflammation. This activates
macrophages which are the system controller. They in turn generate a control
signal in the form of cytokines. The controlled load is the group of local
responder cells. Their output are the elements of histogenesis, which modify
the state of the system, which then feeds back to the loop at the summing
point. Any discussion or research of the collective behavior of a wound must
acknowledge this basic control system. |
|||
|
|
|
||
|
THE PHYSICS AND
PATHOLOGY OF WOUNDS. PART
1. THE WOUND AS A
SYSTEM AND A CONTROLLED MACHINE. Original presentation February 22 - 26, 2010, The John A. Boswick, M.D. Burn and Wound Care Symposium 2010 The presentation and related materials are accessible at: arimedica.com Content may be used for non-commercial educational purposes. Content may not be published or used for commercial purposes
without prior license or permission. Contact information is on the slide. Copyright © 2009, Marc E. Gottlieb, MD Revision 01a, February
22, 2010 |
|||
|
|
|
||
|
These presentations and supplementary
materials are all available at www.arimedica.com. The Physics and Pathology of
Wounds. Part 1. The Wound as a System and a Controlled
Machine. Marc E. Gottlieb, MD, FACS The wound is a transient organ of
inter-operating cells, triggered into being by injury and inflammation, then
extinguishing as it completes its repair of injured stroma. It is a system.
Conventional bioscience tends to characterize properties and interactions of
individual or one-versus-another elements within a system, but physics is
required to understand the integrated timewise behavior of whole systems.
Intrinsic wound pathology and chronicity, and wound failure and therapeutics
are easily explained when wounds are seen as a non-linear System (rather than
as a collection of dual-element linear interactions). For normal wound
physiology and for the pathophysiology of altered and failing wounds, the
governing principles are the physics of complex systems: non-linear N-element
dynamics, control science, population logistics, and self-organizing
automata. Understanding wound physics begins by
characterizing normal wound physiology. The wound is a closed-loop
reference-driven non-linear multicontrol system. Sick and altered wounds have
layers of added complexity, but the quintessential intrinsic machinery of
wound healing – the Wound Module of post-inflammatory wound repair –
functions as just a single control loop. When tissues are injured, the Main
Control Loop of physiological wound repair will drive cells to reorganize
back to a repaired stroma. The wound control system is composed
of these elements: The system state is the open wound and its conditions. It
is compared (?) to a reference, normal epithelialized tissue. Variances
generate an error signal in the form of inflammation. This activates
macrophages which are the system controller. They in turn generate a control
signal in the form of cytokines. The controlled load is the group of local
responder cells. Their output are the elements of histogenesis, which modify
the state of the system, which then feeds back to the loop at the summing
point. Any discussion or research of the collective behavior of a wound must
acknowledge this basic control system. |
|||
|
|
|
||
|
These presentations and supplementary
materials are all available at www.arimedica.com. The Physics and Pathology of
Wounds. Part 2. Auto-Immunopathy and the Intrinsic
Disease of Wound Healing. Marc E. Gottlieb, MD, FACS Many chronic wounds result from
disorders extrinsic to the healing process, e.g. pressure or arterial
disease. What then are the intrinsic diseases of wound healing? Compare the
wound to other organs. The quintessence of heart failure is that it is an
inadequate pump, for lung failure it cannot exchange gases. But the wound is
neither pump and pipes, nor bellows and diffusion membrane, nor is it like
any organ with macro-anatomical structure. It is a transient collection of mutually
interacting self-organizing cells.
Stromal angiocytes and fibroblasts (wound cells) have remarkably few
inherent metabolic or genetic faults. Dysfunction of the aggregate population
is almost always the result of deprivation or predation. Adverse states can
be caused by (1) non-targeted exogenous conditions such as arterial ischemia
or repetitive trauma, and (2) targeted damage directed against these cells
and their structures. As will be presented here, predation against the wound
module is due to a state of auto-immunopathy in which lymphoid cells are
sensitized to wound components. Not only does this occur with classic
connective tissue disorders and other well-recognized auto-immunopathies, but
it happens when a wound becomes intrinsically chronic and pathological. Hypercoagulability and other conditions of
persistent thrombosis and acute inflammation are the underlying states that
induce the auto-immunization. Simply put, intrinsic wound pathology and chronicity
is a dynamical disorder of complex populations caused by auto-immunopathic
disruption of the wound module. In these chronic non-healing wound
samples, the vascular locus is infiltrated with immune cells (left, plasma
cells; middle, plasma cells and eosinophils; right, lymphocytes.) On the left, plasma cells are mixed with
the migratory angiocytes (spindles) that are trying to assemble the
wound. At middle and right, chronic
thrombosis due to a primary hypercoagulable disorder is not only present, it
is the root cause of this entire state. |
|||
|
|
|
||
|
These presentations and supplementary
materials are all available at www.arimedica.com. The Physics and Pathology of
Wounds. Part 3. Chronicity and the Physics of Wound
Failure. Marc E. Gottlieb, MD, FACS The wound module is a transient set of
interacting cells which collectively restore injured tissue to normality, a
fibrous stroma of angiocytes and fibroblasts. Its healthy aggregate behavior
is a well behaved machine, governed by the physics of control systems. A sick
system can result from various extrinsic perturbations, but the core
mechanism of self-sustaining persistent dysfunction, the true intrinsic
disease of wound healing is chronicity itself, the paramount cause being
wound module autoimmunization. This state is disruptive but not fully toxic
or lethal, thus immunopathic wounds have complex behaviors, at times
better-worse-stable-variable, often looking healthy, but always frustrating
as they refuse to cross the finish line. How does one explain such variable behavior
and the differences between normal and chronic-and-pathological (cap) wounds? Simply stated, intrinsic wound
pathology and chronicity is a dynamical disorder of complex populations. The
physics governing complex behaviors in complex systems is non-linear dynamics
(nld). In addition to control, three aspects of NLD are especially important
to wound pathology. (1) Population logistics. Healthy healing is a sequence
of one-shot self-completing linear events: primary injury & thrombosis
–then– acute inflammation –then– wound module. Pathology creates abnormal
population dependencies (nutrition, starvation, predation, cultivation) and a
new population, chronic inflammation. Non-linear perpetual complexity arises
in the logistics of injury & thrombosis –vs– acute inflammation –vs–
wound module –vs– chronic inflammation –vs– injury & thrombosis. (2)
Cellular automata & self-organization. The “cellular” agents of the wound
module (real biological cells in this case) have a small set of deterministic
rules of interaction with each other.
When allowed to function properly, stromal rebuilding is automatic and
correct. Under pathological
conditions, self-organization, i.e. wound healing is disrupted. (3) Chaos & N-body dynamics. The net
effect is that the wound, a set of several interacting cell populations, has
3 attractors (basins, dynamically stable states or behaviors): convergence
(healing), divergence (ulcerating), and self-sustained chaotic orbits
(chronicity). Basic methods to demonstrate
non-linear dynamics: left, the logistical map of competing populations;
middle, diffusion-limited-aggregation, an example of self-organizing
automata; right, attractors and chaos in the Mandelbrot set of complex-plane
iteration. While seemingly abstract,
these structures are directly correlated with wound events. |
|||
|
|
|
||
|
|
|||
|
|
|
||